NNadir
NNadir's JournalAI Searches of the Scientific Literature to Make Discoveries in Materials Science.
The paper I'll discuss in this post is this one: Unsupervised word embeddings capture latent knowledge from materials science literature (Tshitoyan, et al, Nature 571, 95–98 (2019))
Newton's Principia lay in a drawer, the story goes, unpublished for years, until Edmond Halley, of comet fame asked Newton for help with Kepler's laws, the mathematics of which Newton had already solved. This, and not the comet, was Halley's greatest discovery, what Newton had already done but not bothered to publish.
It was entirely possible that Newton might have died with his discovery unknown, and although many facets of what he discovered might have been discovered independently, because of the basic truths behind them - Halley was himself working the problem - history would have been very different without the publication of Principia Mathematica; it is a book that changed the world.
I expect that many scientists died with potentially important work unpublished; in Newton's case, chemistry might have evolved more quickly were he not ashamed of his Alchemical work which he kept hidden.
Other works are published, forgotten and then rediscovered years after the fact and become important. Probably the most famous case was Gregor Mendel's famous work on genetics was forgotten until well after he died in 1884. His work was rediscovered by early geneticists - working without any knowledge of DNA - in the early 20th century: Happily they ultimately acknowledged his priority.
I have made it a habit over the last three decades to spend much of my free time, 5 to 10 hours a week, sometimes much more, in scientific libraries. I try to spend some, but cannot spend all of this time, in desultory reading, unconnected with my professional work.
Over the last several years I've developed a habit, probably unwise, of paying more attention to papers that are highly cited as opposed to those that aren't, particularly when I am addressing a particular question that has arisen in my mind.
Here, for instance, is an excellent paper that has only been cited by 10 people in the last 11 years: Reactor physics ideas to design novel reactors with faster fissile growth (Jagannathan et al Energy Conversion and Management Volume 49, Issue 8, August 2008, Pages 2032-2046) Four of the ten citations are the author citing himself, but only recently, in 2019, has the paper been "rediscovered."
There are certain journals that I at least scan religiously, they are overly represented in my posts on this website. There are many other journals that I wish I had time to read. There are several journals that attempt to give broad overviews, "review" journals, and even journals that solicit scientists to discuss their own work.
Here is a video by Dr. Cynthia Burrows of the University of Utah, editor of Accounts of Chemical Research giving the raison d'être for her journal, which as that scientists can simply not read all of the journals that they would like to read:
Young scientists will have tools for sorting through the prodigious scientific literature that we didn't have years ago. I remind my son of this point frequently, asking him to imagine a world without Google Scholar, Scopus, CAS and Scifinder.
Of course for a tool to be valuable, you have to use it, which I expect he does.
This brings me to the paper I cited above, which has a very interesting approach, using lexographic programming to sort through broad swathes of the scientific literature to direct scientists to work that may be related in such a way as to allow for the discovery of new materials.
From the introduction/abstract:
...Assignment of high-dimensional vectors (embeddings) to words in a text corpus in a way that preserves their syntactic and semantic relationships is one of the most fundamental techniques in natural language processing (NLP). Word embeddings are usually constructed using machine learning algorithms such as GloVe13 or Word2vec11,12, which use information about the co-occurrences of words in a text corpus. For example, when trained on a suitable body of text, such methods should produce a vector representing the word ‘iron’ that is closer by cosine distance to the vector for ‘steel’ than to the vector for ‘organic’. To train the embeddings, we collected and processed approximately 3.3 million scientific abstracts published between 1922 and 2018 in more than 1,000 journals deemed likely to contain materials-related research, resulting in a vocabulary of approximately 500,000 words. We then applied the skip-gram variation of Word2vec, which is trained to predict context words that appear in the proximity of the target word as a means to learn the 200-dimensional embedding of that target word, to our text corpus (Fig. 1a). The key idea is that, because words with similar meanings often appear in similar contexts, the corresponding embeddings will also be similar...
...For example, many words in our corpus represent chemical compositions of materials, and the five materials most similar to LiCoO2 (a well-known lithium-ion cathode compound) can be determined through a dot product (projection) of normalized word embeddings. According to our model, the compositions with the highest similarity to LiCoO2 are LiMn2O4, LiNi0.5Mn1.5O4, LiNi0.8Co0.2O2, LiNi0.8Co0.15Al0.05O2 and LiNiO2—all of which are also lithium-ion cathode materials.
Some pictures from the text:
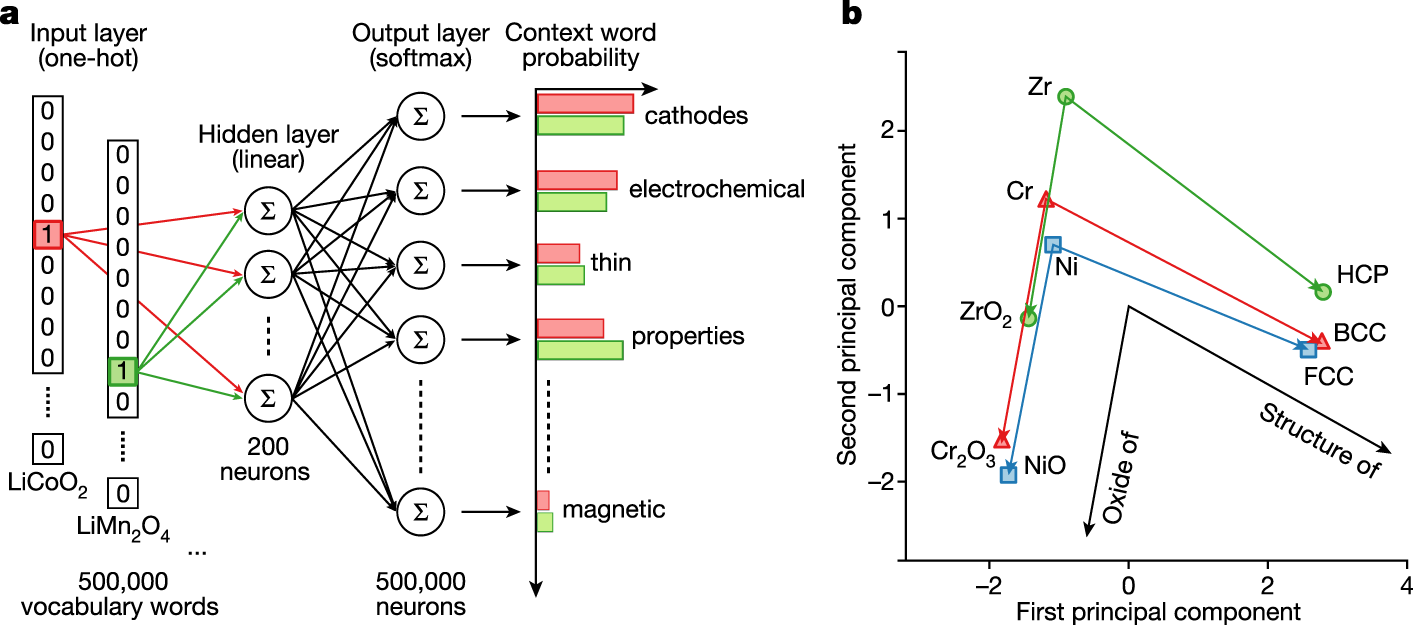
The caption:

The caption:

The caption:
(I have personally been very interested in thermoelectrics because of their potential to produce electricity, free of mechanical systems from the heat of used nuclear fuels, and heat transfer lines during rejection of heat to the environment, raising the thermodynamic efficiency as well as doing things like engineering away the potential for another event like Fukushima.)
From the conclusion of the paper:
Cool paper, I think.
A very different approach to knowledge, rediscovering that which already exists.
Have a nice weekend.
More of the same: An exciting boost for solar cells!!!!!!!!
The paper I'll discuss in this post is this one: Sensitization of silicon by singlet exciton fission in tetracene (Baldo et al, Nature. 571, 90–94 (2019). The paper is probably not open sourced but the news item discussing it may be: An exciting boost for solar cells. I took the title of this post from the news item adding my own editorial comment to it: I have convinced myself that so called "renewable energy," as popularly imagined, is useless as strategy to address climate change, even though the delusional faith - and inasmuch as it is delusional it is also pernicious - persists that it will do so. It has not done so. It is not doing so. It won't do so.
Of course, as a political liberal, I was certainly not immune to believing it would do so, and years ago I certainly would have applauded the idea of spending trillions on it, which we have done, but the result of this experiment is written in the planetary atmosphere:
Up-to-date weekly average CO2 at Mauna Loa
Week beginning on June 23, 2019: 413.35 ppm
Weekly value from 1 year ago: 410.73 ppm
Weekly value from 10 years ago: 388.54 ppm
Last updated: July 4, 2019
There is, as many of us know, but certainly not all of us, a big, big, big difference between belief and fact.
In science, if there is a theory that disagrees with the experimental result, the theory goes, not the experimental result. Again, the measurement of the experimental result is in, as measured above at Mauna Loa.
My whole adult life - and I'm not young - I've been reading all about "breakthroughs" in so called "renewable energy," having read many of them in the E&E forum right here. I'm sure if you mosey over there, you can still read them.
The news item includes text that features the bad thinking that has gotten us into this nearly or totally intractable mess of climate change. It is these sentences:
The first sentence is, by the way, true. All agricultural energy - including human and animal muscle power - derives from the sun. This should inspire some critical thinking, but generally in popular discourse doesn't. There is a reason that humanity abandoned "renewable energy" in the 19th century, as I often state. The reason is that most people lived short, miserable lives of dire poverty. In fact, if one cares to look, this is still a factor: Slightly less than half of the 7 million people who die each year from air pollution are in fact killed by the waste products of biomass combustion (cigarettes not included here) and overwhelmingly these are poor people who live much as people might have lived hundreds of years ago.
The second sentence, the one including that magical word so badly abused by advocates of so called "renewable energy," "watts" is rather disgusting, since it implies that there is plenty of energy from the sun and the "only" thing that we need to do is to collect it. This is garbage thinking, although advocates of what I personally regard as the only environmentally acceptable and sustainable form of energy, nuclear energy, were quite nearly destroyed by the application of such thinking.
To wit:
A kg of uranium, when transmuted into plutonium, contains about 80.2 trillion Joules of energy. This is roughly the energy equivalent of 620,000 gallons of gasoline. The world energy demand as of 2017, as reported by the international energy agency, was 584 exajoules, which corresponds to average continuous power of 18.5 trillion watts. Thus all of the world's energy demand could be met by fissioning about 230 grams of plutonium each second.
When I was a kid, pre-adolescent, my mother, a completely uneducated woman who was nonetheless interested in my education, bought me a "toy" that one almost certainly not buy today, a small eye piece that if I held it to my eye in a closet allowed me to "see" radioactivity. I spent hours with that thing and I recall that the description from the notes that came with the thing of the flashes that I saw while sitting in a dark closet, fascinated, said that I was seeing individual atoms decay.
It was probably true. I suspect that the toy consisted of a phosphorescent screen impregnated with radium (which I had on my boyhood clock in much larger amounts) or polonium or some such isotope. The toy lead me to believe that "the atom" contained enormous amounts of energy, which of course it does.
However, as we all know, and as we are often reminded by anti-nuke appeals to stupidity and ignorance, it is not the energy content of plutonium that matters; what matters is the cost - and I would argue - the environmental impact of the device for collecting the energy that matters. Even though the United States built more than 100 nuclear reactors in the period between 1957 and 1980, using technology developed in the 1950's and 1960s, while providing some of the cheapest electricity on Earth, this while saving human lives that would have otherwise been lost to air pollution, we now hear that nuclear energy is "too expensive." In other words, what has already happened is now considered impossible.
Well then...
The same that applies to the nuclear industry - the cost of the device as opposed to the total quantity of energy - applies to the solar industry, it is the device that matters, not the energy flux of solar energy, and as the device matters, so does its environmental impact, which is largely ignored whenever the issue of so called "renewable energy" is discussed in a rote - but in my view nonsensical - evocation in which it is routinely described as "green." The word "green" of course, has come to mean "sustainable" and "environmentally benign" which the solar industry is decidedly not. Since the solar industry cannot produce continuous energy - it is widely reported and generally accepted that the sun goes "down" every evening - any discussion of its cost in terms of its internal - dollars per unit of energy paid by the consumer - costs and its external costs - the environmental destruction its use incurs and which is charged to all current and future generations - should accrue to solar energy, but since, as we are accustomed with and happy to lie to ourselves, is not. A battery is an energy wasting device, per the second law of thermodynamics, and overall batteries are not sustainable devices since their environmental cost is not acceptable or sustainable.
So this brings me to the paper under discussion, the solar "breakthrough" du jour.
From the news item:
The first sentence, as anyone who has lived as long as I have will have listened to half a century of cheering like madmen and madwomen for solar energy, should give some pause for critical thinking, but it won't.
The rest of this excerpt is nonsense, except for the fact that "...researchers are continually driven to make these devices..." which is largely a function of grant money, a cultural rather than technological imperative. (Scientists are not immune from culture; the are in fact a part of culture and what they do is a function of culture.)
Now let's turn to the paper itself, which has some very interesting science, even though the application of this science is not what it would seem from the description in the news item.
From the abstract, as Nature often uses abstracts as part of the content:
Sounds great!!!
From the main text:
As I noted recently in a recent post here in connection with the "miracle" cesium lead iodide perovskite solar cells, lead, a toxic element in most places becomes instantly "green" when attached to the word "solar."
Figure 1:

The caption:
To continue with the text:
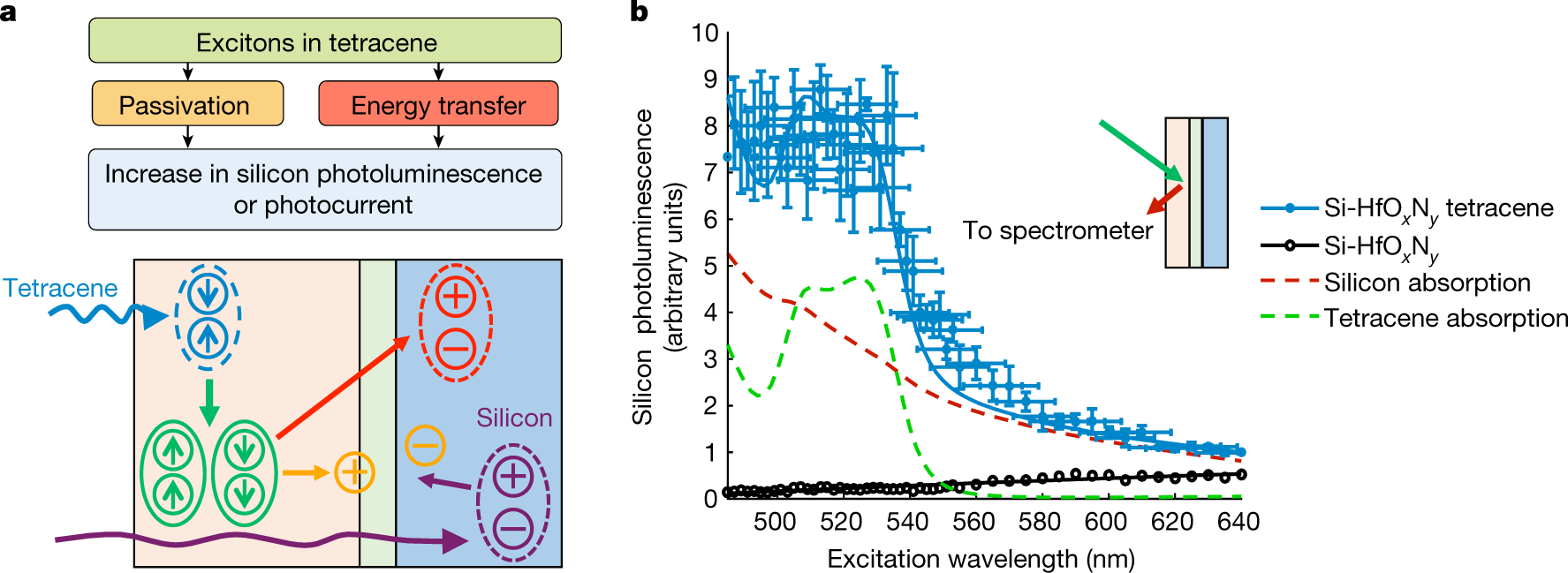
The authors discuss the need for precise control of the thickness of the passivation layer, essentially the construction of a layer on an atomic scale. This is because the transfer of electrons depends on quantum mechanical tunneling, which decreases with the thickness of the layer through which it must "tunnel." The interesting approach involves the remarkable advances in nanotechnology, in this case, atomic layer deposition. For this purpose they chose to utilize hafnium oxynitride to make this layer since its deposition properties on silicon is well understood and controllable.
Although solar energy is magically green, the scientist in me, the skeptic on how "green" all of this really is was inclined to view the processing described herein:
For a schematic of the fabrication process, see Extended Data Fig. 2. We purchased silicon wafers from PureWafer (for details see Supplementary Table 3) and transferred them to a clean room. We then performed a standard Radio Corporation of America (RCA) clean as follows. First, organic contaminants were removed by immersing the sample in a 5:1:1 ratio solution of deionized water, aqueous ammonium hydroxide (29%), and aqueous hydrogen peroxide (30%) for 20 min at 80?°C. Second, the native oxide was removed by etching with aqueous hydrofluoric acid (1%) for 60 s. Third, metal-ion contaminants were removed from the wafer surface in a 5:1:1 ratio solution of deionized water, aqueous hydrochloric acid (37%), and aqueous hydrogen peroxide (30%) for 20 min at 80?°C. We then deposited 10 nm of aluminium oxide on both sides using atomic layer deposition. Using photolithography, we fabricated electron-selective contacts (aluminium/lithium fluoride) and hole-selective contacts (aluminium/molybdenum oxide) in an interdigitated fashion. We then diced the wafers into chips and protected the back side of the individual chips using PDMS and a second piece of encapsulating silicon to provide a seal. We experimentally verify in Extended Data Fig. 10 that a full RCA clean before HfOxNy deposition is not compatible with our solar cell fabrication because the protective rear PDMS seal decomposes in the presence of acids, and the monomers subsequently reattach to the front side by condensation27,28. Consequently, to minimize contamination by PDMS in this process, we cleaned the front side using 10% aqueous hydrofluoric acid for 1 min and a 5:1:1 ratio solution of deionized water, aqueous ammonium hydroxide (29%) and aqueous hydrogen peroxide (30%) for 20 min at 60?°C. We fabricated the downconversion front side as detailed below and then peeled off the silicon protecting the back contacts (as shown in Extended Data Fig. 2).
PDMS is polydimethylsiloxane. It is made by passing chloromethane over silicon, the silicon having been produced by reduction with carbon.
If one is an environmentalist, in my opinion, as opposed to a person engaged in mindless hand-waving and wishful thinking, it useful to look at these lab scale processes and to consider what might be involved in scaling them up to a scale that mattered. It is also useful to consider that right now, in 2019, with concentrations of the dangerous fossil fuel waste carbon dioxide having reached 415 ppm this year in the atmosphere, the entire so called "renewable energy" industry is trivial. Combined, solar and wind and geothermal and tidal energy produced, as of 2017 less than 11 exajoules of the 584 exajoules humanity generated and consumed that year.
Anyway the hafnium oxynitride layer on silicon works great! (In the lab...)
Some pictures from the paper:

The caption:
Another graphic:
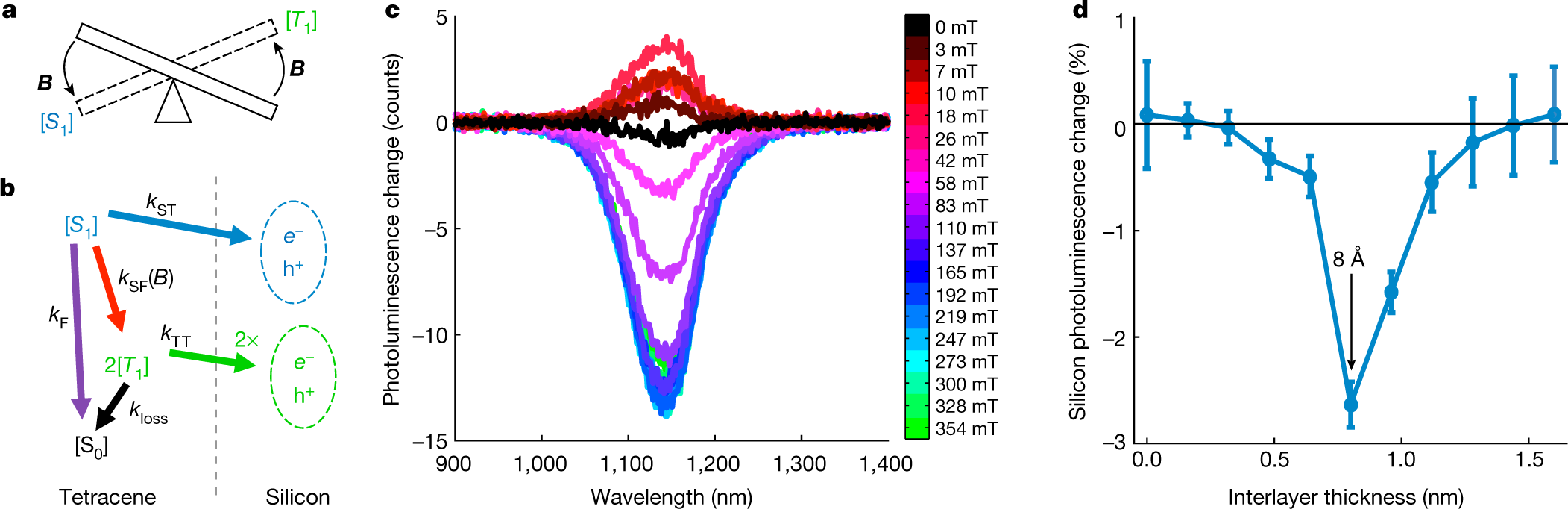
The caption:
Simplistic..simplistic...
We have simply bet the future of humanity - of all future generations - on the ability of so called "renewable energy" to save the day. Are we tired of all this "winning?"
Anyway:
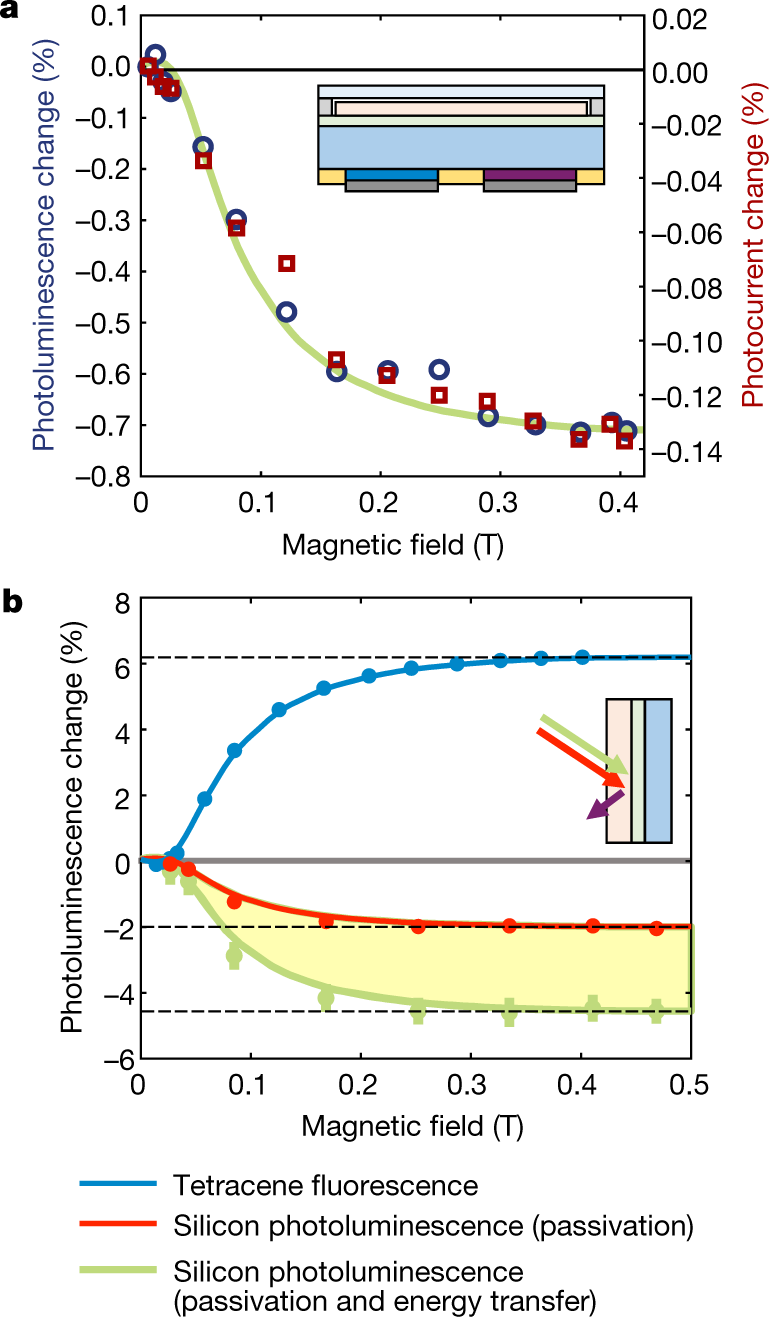
The caption:
The down conversion of high energy photons to photons in this useful bandwidth is most interesting. High energy radiation is generally available: We can make all we want although in general we are too stupid to want to make it even though there is an excellent argument that we need it.
As for this latest "renewable energy" "breakthrough," it offers a useful exercise in understanding how "renewable" so called "renewable energy" actually is.
Hafnium is found as an impurity in all zirconium ores, generally at a concentration (with respect to zirconium) of around 3%, and must be removed from them in order to utilize zirconium in nuclear reactors. Hafnium free zirconium is also obtainable as a fission product, but very little hafnium (if any) is synthesized in nuclear reactors, although one can imagine tiny amounts being made in long lived "breed and burn" reactors that can run for decades without refueling.
Hafnium is considered an "endangered" element, one that may be totally depleted for future generations:

One could argue that the amount of hafnium in these solar cells is tiny, but that claim will not stand up to an effort to scale this low energy to mass type system to a scale of tens of exajoules. Of the less than 11 exajoules from so called "renewable energy" as a whole, solar energy is trivial in the trivial system with respect to wind energy, which represents the bulk of the 11 exajoules.
Nor is this chemistry remotely clean. Chloromethane is made from dangerous natural gas, and hydrofluoric acid is not good for you. Although I am personally fond of hydroflouric acid's use in nuclear processes, these processes require relatively trivial amounts, since the energy to mass ratio of nuclear energy is huge, whether compared to gasoline, to coal and to dangerous natural gas or to far less concentrated forms of energy, the mass intensive, and thus environmentally suspect solar industry.
Have a nice "4th of July" evening. Don't get burned by fireworks.
What It Would Really Take to Reverse Climate Change.
Recently, although I'm no longer an MSR/Thorium kind of guy - although I'm very fond of fluid nuclear reactors of other types - I've been reading this book: Thomas Dolan, Ed., Molten Salt Reactors and Thorium Energy.
It's quite a nice book for general concepts certainly not strictly limited to thorium and MSR's, but includes very nice little riffs on things like the equations of state for liquids, statistical mechanics and thermodynamics, and obscure points, like what is described as a "small deviation" nonadjoint properties of matrices applied to model MSR's using Fick's law as applied to the diffusion of neutrons.
It's a pleasure to read, a desultory tour of physical science as applied to nuclear reactors, and very applicable beyond FLIBE (or similar to FLIBE) based MSRs.
Of particular pleasure is the chapter (Chapter 2) written by the MIT Nuclear Engineer/Chemical Engineer/Materials Science Engineer par excellence Charles Forsberg, whose work and writings I admire enormously.
In a brilliant discussion of the economics of so called "renewable energy" and carbon free energy in general, Forsberg makes some points that I hadn't actually thought much about, although I've long been aware that the real result of the expansion of so called "renewable energy" is to permanently entrench the use of dangerous fossil fuels.
(Note: Forsberg is in no way as hostile to so called "renewable energy" as I am.)
Of course, as a general text, a desultory general text with multiple authors, the real value is in the references.
One of the references in the book is available open sourced on line, and is written by two Google Engineers who worked on the abandoned RE<C project, which was designed to accelerate the 100% renewable energy scheme that has not worked, is not working and will not work but which they believed would work.
It's an interesting read as well.
Some excerpts:
Starting in 2007, Google committed significant resources to tackle the world’s climate and energy problems. A few of these efforts proved very successful: Google deployed some of the most energy-efficient data centers in the world, purchased large amounts of renewable energy, and offset what remained of its carbon footprint.
Google’s boldest energy move was an effort known as RE<C, which aimed to develop renewable energy sources that would generate electricity more cheaply than coal-fired power plants do. The company announced that Google would help promising technologies mature by investing in start-ups and conducting its own internal R&D. Its aspirational goal: to produce a gigawatt of renewable power more cheaply than a coal-fired plant could, and to achieve this in years, not decades.
Unfortunately, not every Google moon shot leaves Earth orbit. In 2011, the company decided that RE<C was not on track to meet its target and shut down the initiative. The two of us, who worked as engineers on the internal RE<C projects, were then forced to reexamine our assumptions...
...As we reflected on the project, we came to the conclusion that even if Google and others had led the way toward a wholesale adoption of renewable energy, that switch would not have resulted in significant reductions of carbon dioxide emissions. Trying to combat climate change exclusively with today’s renewable energy technologies simply won’t work; we need a fundamentally different approach...
...We’re hopeful, because sometimes engineers and scientists do achieve the impossible. Consider the space program, which required outlandish inventions for the rockets that brought astronauts to the moon. MIT engineers constructed the lightweight and compact Apollo Guidance Computer, for example, using some of the first integrated circuits, and did this in the vacuum-tube era when computers filled rooms. Their achievements pushed computer science forward and helped create today’s wonderful wired world. Now, R&D dollars must go to inventors who are tackling the daunting energy challenge so they can boldly try out their crazy ideas. We can’t yet imagine which of these technologies will ultimately work and usher in a new era of prosperity—but the people of this prosperous future won’t be able to imagine how we lived without them.
The link to the full text is here, on the IEEE website: What It Would Really Take to Reverse Climate Change.
I personally believe that the technology to reverse climate change is just on the edge of feasibility, but our cultural biases - and I'm including our biases on the political left as well as those on the political right - prevent us for taking a responsible attitude to future generations in particular and life on Earth in general.
In this sense, although at the end of my life I'm losing hope, I admire the statement in the last excerpted paragraphs, because, while left and right we are in an age of contempt for scientists and engineers - I still think it just possible that, as the authors state, "Sometimes engineers and scientists do achieve the impossible."
I trust you're enjoying the holiday, and I'm sure most of you will not be watching the banana dictator's parade for morons.
Nature (News): The Reality Behind Perovskite Solar Cells.
The news item I'll discuss in this post comes from one of the world's most prominent scientific journals Nature. I believe it's open sourced. It's this one: The reality behind solar power’s next star material (Nature 570, 429-432 (2019))
Some excerpts:
...More than a dozen companies worldwide (see ‘Solar hopes’), a mixture of established electronics giants and start-ups, are hoping soon to sell panels made with perovskites. Dozens more are involved in making materials for the products, says Margareth Gagliardi, an analyst with BCC Research in Wellesley, Massachusetts.
For decades, slabs of crystalline silicon have dominated the solar industry. Other materials that can be layered in thin films, such as copper indium gallium selenide (CIGS) and cadmium telluride (CdTe), have captured less than 5% of the market, because it’s hard to make them as efficient or cheap as conventional solar panels. Perovskites could be a different story. They should be cheaper to make and seem impressively efficient at converting sunlight into electricity — in the laboratory, at least.
Wow. For decades...
Often over the last ten years since I went from enthusiasm for the solar industry to hostility, as I've remarked on the failure of the solar industry to do anything to address climate change after half a century of wild cheering for it, one of the more common explanations (excuses?) is that it's a "new industry."
It's a "new industry" that has soaked up over a trillion dollars (with another more than a trillion on wind) in the last ten years.
Frankfurt School/UNEP Global Renewable Energy Investment, 2018, Figure 3, page 14
Further excerpts:
If...if...if...
I can't say how many "ifs" I have heard about solar energy since 1970 (when I was a kid), soon to be 50 years ago.
Actually we have put all of our "eggs in one basket" in addressing - or put more properly pretending to address climate change: So called "renewable energy."
I note, with tremendous sadness at the edification of fear and ignorance, that many of the people who have bet the planetary atmosphere on so called "renewable energy" are more opposed to nuclear energy than to dangerous fossil fuels, even though dangerous fossil fuel waste, along with biomass combustion waste is responsible for 7 million deaths per year, and so called "nuclear waste," um, isn't.
Here is the most recent full report from the Global Burden of Disease Report, a survey of all causes of death and disability from environmental and lifestyle risks: Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 (Lancet 2016; 388: 1659–724) One can easily locate in this open sourced document compiled by an international consortium of medical and scientific professionals how many people die from causes related to air pollution, particulates, ozone, etc.
The note about lead being in these solar cells of course doesn't matter, or course, anymore than cadmium or tellurium mattered since no amount of toxic material being distributed by the solar industry can matter since solar cells are always "green," as we often hear in all kinds of advertisements about being "green."
Except they aren't. The last major distributed energy scheme involving lead was tetraethyl lead in gasoline, in the world's most successful "distributed energy" technology, the automobile. Up until the present day, whenever the chaparral in California blazes, some of that historically deposited lead is volatilized.
Now we want to distribute more lead. Good idea!
A picture from the article.
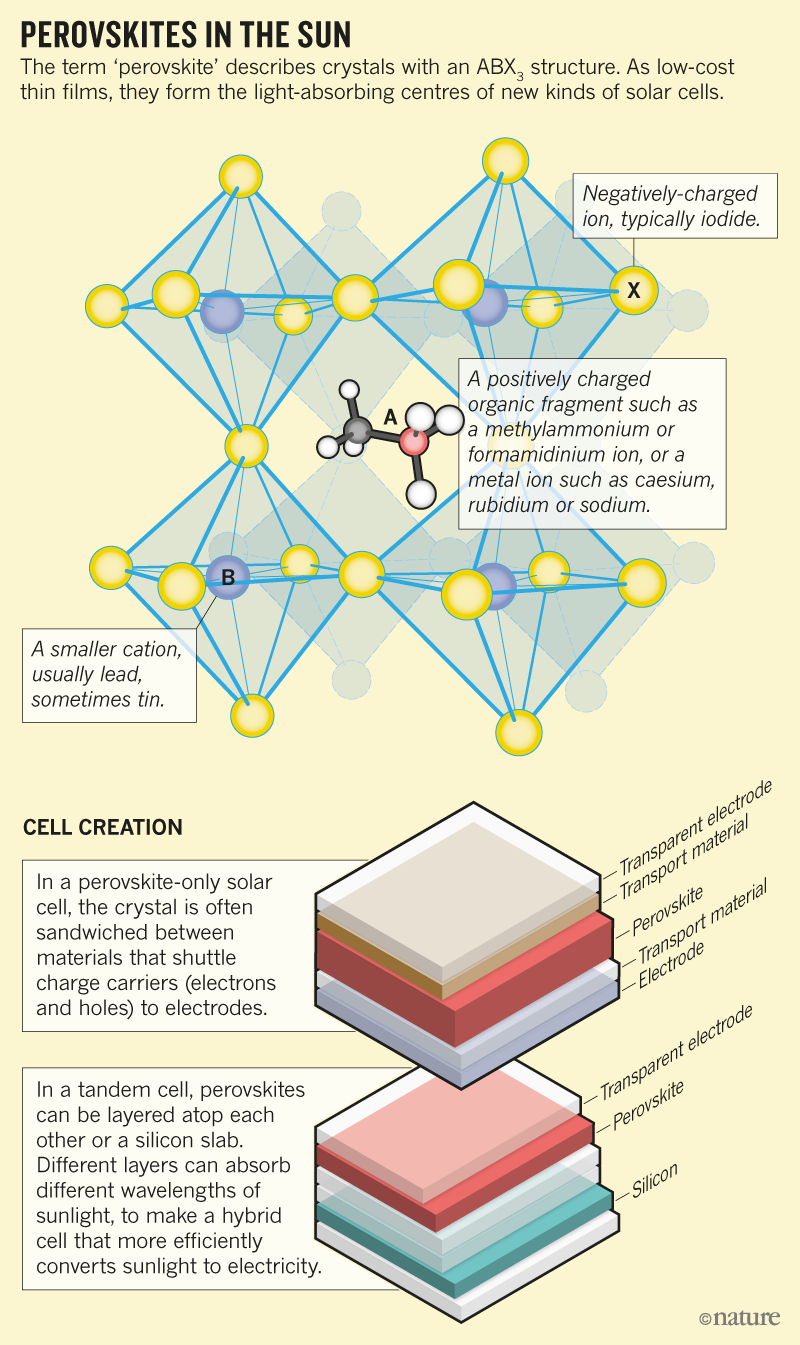
One may also question how "renewable" cesium is, and for that matter, iodine, but no matter...
No matter...
No matter...
More:
The major challenge for perovskites, however, is whether they can last as long as silicon panels, which generally come with a 25-year warranty. Perovskite stability “needs to approach the norms established by silicon” and that is “now looking increasingly unlikely”, says Martin Green, who researches perovskites and other solar materials at the University of New South Wales in Sydney, Australia. His team collaborates on the materials with two large Chinese solar-panel makers, Trina Solar and Suntech...
...Perovskites are sensitive to air and moisture, but that shouldn’t be a killer problem. Commercial solar panels already encapsulate their photovoltaic materials in plastic and glass for protection...
...A deeper issue lies in the crystals themselves. In some cases, the structures shift as the perovskites warm up; the change is reversible, but it affects performance...
Ummmm... plastic...eating it is good for you, and be sure you are eating it, pretty much every damned day, a little more won't hurt you.
And the argument is made that a little more lead won't hurt you, since possibly lead based solar cells are even greener than silicon solar cells:
Another potential stumbling block for perovskite cells is that the best of them contain lead, a toxic metal. Researchers have tried alternatives, such as tin, but performance declines. That doesn’t mean the cells can’t be used. A life-cycle analysis of Oxford PV’s tandem cells suggests that the small amount of lead they contain wouldn’t have much impact on environmental toxicity if it leaked. The analysis also argues that silicon cells have a worse overall environmental impact because of the resources used in their production.
Whiny people disagree:
We can, of course, compromise on anything declared in the popular imagination, if not reality, to be "green."
The result of the bet of the planetary atmosphere on so called "renewable energy" has been reported by the International Energy Agency:
In this century, world energy demand grew by 164.83 exajoules to 584.95 exajoules.
In this century, world gas demand grew by 43.38 exajoules to 130.08 exajoules.
In this century, the use of petroleum grew by 32.03 exajoules to 185.68 exajoules.
In this century, the use of coal grew by 60.25 exajoules to 157.01 exajoules.
In this century, the solar, wind, geothermal, and tidal energy on which people so cheerfully have bet the entire planetary atmosphere, stealing the future from all future generations, grew by 8.12 exajoules to 10.63 exajoules.
10.63 exajoules is under 2% of the world energy demand.
2018 Edition of the World Energy Outlook Table 1.1 Page 38 (I have converted MTOE in the original table to the SI unit exajoules in this text.)
But the most telling result of this bet is written in the planetary atmosphere. From this week's data from the Mauna Loa Carbon Dioxide Observatory:
Up-to-date weekly average CO2 at Mauna Loa
Week beginning on June 23, 2019: 413.35 ppm
Weekly value from 1 year ago: 410.73 ppm
Weekly value from 10 years ago: 388.54 ppm
Last updated: July 1, 2019
If the fact that the carbon dioxide concentrations are 24.81 ppm higher than they were ten years ago troubles you, don't worry, be happy.
It's not your problem; it's a problem for babies, since it is they and not us, who will have to live up to all that "by 2030" and "by 2050" bullshit that they've been handing out at the anti-nuke ignorance squad at Greenpeace ever since 1970 when, of course, it was "by 2020," but no matter...
No matter...no matter...
Elon Musk. Tesla electric car. Plastic and steel wind turbines in the benthic ecosystem off New Jersey. Solar thermal. Solar house. Solar roof. Happy. Happy. Be happy. Be nice.
Don't worry...be happy...
No one now living, Bill McKibben and his Prius be damned, will ever see a reading at Mauna Loa of below 400 ppm of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere again.
History will not forgive us, nor should it.
Have a nice evening.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,515