NNadir
NNadir's JournalToward Sustainable Biologically Derived Anodes for Aluminum Production.
The paper I'll discuss in this post is this one: Synthesis and Characterization of Bio-pitch from Bio-oil (Ying Lu, Dazhi Li, Xianai Huang, Donald Picard, Roozbeh Mollaabbasi, Thierry Ollevier,* and Houshang Alamdari* ACS Sustainable Chem. Eng. 2020, 8, 31, 11772–11782)
The Hall-Heroult process responsible for the production of aluminum metal from alumina, Al2O3, is electrolytic in nature, which theoretically should result in the production of oxygen, but doesn't in practice, do so. In real practice, even though the reaction is most definitely driven electrochemically, the oxidized species does not represent oxide being converted to oxygen gas, but rather represents the oxidation of carbon to give carbon dioxide:
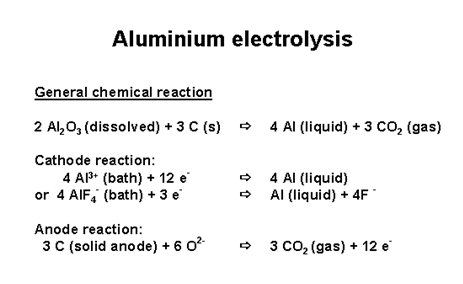
European Carbon and Graphite Association.
Under certain conditions, side products are formed, the greenhouse gas CF4, for example where fluorine source is the flourine gas released from the synthetic cryolite in the molten salt bath, and carbon monoxide, from the Boudouard reaction between carbon dioxide trapped in pores of the anodes and the anode carbon itself. (Other minor gases include tetrafluoroethylene) etc.
According to the information from the World Aluminum Institute the world produced 63,697,000 metric tons of aluminum in 2019. The amount of electricity consumed, also as reported by the World Aluminum Institute to produce aluminum in 2019 was 848,845 GWh. (Accessed 8/17/20) More than half of the world aluminum supply was produced in China - and despite all the bull you can read around here, say over at the E&E forum about how coal is dead - China still burns massive amounts of coal. The World Aluminum Institute, thus reports that of the 848,845 GWh reported to produce aluminum, 509,393 GWh was produced by burning coal. A good working figure for the carbon intensity of electricity produced by coal is 1,100 grams of carbon dioxide are produced for each kwh, meaning that the electricity generated by coal combustion alone in order to drive aluminum plants was 560,000,00 million tons.
This of course, excludes the carbon in the anodes, which are made with coal tar pitch and petroleum coke.
From the equation above, and the atomic weight of aluminum, 28.96 grams per mole, and the molecular weight of carbon dioxide, 44.095 grams per mole, and the ratio - at least at 100% yield (which is unlikely) - of 3 moles of carbon dioxide being produced for 4 moles of aluminum, we can see that 63,697,000 metric tons of carbon dioxide, one can easily calculate that the oxidation of the electrodes added about 27 million tons of carbon dioxide to the atmosphere.
Aluminum production for various technical reasons I will not discuss here, needs to be produced in continuous processes. Although a tiny amount of power was reported for so called "renewable energy" generated electricity by the World Aluminum Institute's 2019 figures, 23,099 GWh, or in the "percent talk" that advocates of so called "renewable energy" love so much, 2.7% of the total electricity produced. Since aluminum plants are required to operate continuously, this 2.7% probably represents almost in its entirety, the Icelandic production using geothermal energy, supplemented by hydroelectricity.
It is not possible to produce reliable continuous power using the much ballyhooed, but entirely ineffective, wind and solar industry, and therefore here, as elsewhere, it is not really possible to displace coal with wind and solar energy, despite so much rhetoric - all of it delusional on a Trumpian scale - to the contrary.
It is, of course, possible to produce continuous power using nuclear energy, which unlike wind and solar can displace nuclear, but despite this fact, nuclear energy contributed only 13,828 GWh to aluminum production, dominated by Europe and China. Hydro is a major player in aluminum production, producing about 40% as much electricity as coal does for this purpose (210,154 GWh) but we are fresh out of rivers to destroy for electricity generation. It does seem that the continual rise in anti-nuke fear and ignorance is slowing down a bit, and may be peaking, but the reality is that nuclear energy has been prevented from reaching its potential by successful appeals to fear and ignorance, and thus aluminum production on this planet is responsible for about 2% of the 35 billion tons of carbon dioxide humanity releases each year.
The point of this diatribe is that the amount of carbon that can be saved using the technology being explored in this paper, the displacement of coal tar pitch with biooil pitch is small, only about 27 million tons, but perhaps a worthwhile object of consideration, since the other metal that uses prodigious amounts of coke is the steel industry. Moreover, considerable amounts of carbon are present in steel alloys, meaning that this carbon in those alloys is sequestered.
Nevertheless, it is important to keep scale in mind when discussing climate change, but too often we don't. This explains why people can prance around obliviously pretending that solar and wind energy matter, when in fact they don't: The failure to appreciate scale.
From the paper's introduction, covering some of the ground I've discussed above:
Biomass is a well-known renewable, sustainable, and environmentally-friendly carbon source. Consequently, it could be a potential alternative source to produce binder for carbon anode making.(4,5) The pyrolysis of biomass can produce solid bio-carbon, liquid bio-oil, and a gas phase, which is the result of chemical reactions involving the molecular breakdown of large molecules into smaller ones at high temperature and in the absence of oxygen. Attributable to the removal of oxygen-rich volatile matters of biomass during the pyrolysis process, the resultant products have a higher heating value.(6)
The "oxygen-rich volatile matters" are probably dominated by methanol, which years ago, when I was a kid, used to be sold in hardware stores as "wood alcohol." Almost all of the methanol now produced on earth is not from the destructive distillation of wood, but rather by the partial oxidation of dangerous natural gas's methane component, or else hydrogenation of carbon dioxide (or monoxide) using hydrogen produced by the reformation of dangerous natural gas.
What the authors explore here is a particular approach to converting "biooil" - the subject of much discussion in the scientific literature - into "biopitch" to be used as a binder to make anodes for Hall-Heroult aluminum production reactors:
The properties are described as such:
"CTP" here is "coal tar pitch."
A brief description of "biooil" is provided:
The process utilized in the paper is vacuum pyrolysis, heating the biooil in a vacuum at different temperatures and for different periods of time.
They purchased the biooil from a supplier. Reportedly the biomass source was largely softwood sawdust, 80% pine, 20% cedar.
The experimental conditions are briefly described as such:
Table 1:

"Coking Value" is a property defined by the fractions extracted into various solvents: Batia [iet al., Journal of Materials Science volume 22, pages3847–3850(1987)]
Some pictures from the text:

The caption:

PAH's (polyaromatic hydrocarbons, aka, PNA (polynuclear hydrocarbons) are the most carcinogenic components of coal tar:
The caption:

The caption:

The caption:
MALDI is "Matrix assisted laser desorption ionization," a mass spectrometry technique for determining the structure of molecules.

The caption:

The caption:

The caption:

The caption:

From the conclusion:
...Finally, a hypothetical reaction mechanism of bio-pitch synthesis from bio-oil was proposed on the basis of the chemical analysis of bio-oil and bio-pitch. The physical properties of the bio-pitch were characterized and compared to those of coal-tar-pitch. Compared to coal-tar-pitch, all bio-pitch samples exhibited much lower PAHs, quinoline insolubles, and sulfur contents, representing significant health and environmental advantages as a binder in the anode formulation. It was shown that some distillation conditions, i.e., temperature, heating rate, and pressure, are important parameters to adjust the softening point of the bio-pitch and its viscosity, both being important anode manufacturing parameters. Further investigation is required to evaluate other characteristics of bio-pitch in order to develop a new and environmentally-friendly alternative binder in the anode formulation and to confirm its appropriateness for replacing coal-tar-pitch...
From my perspective, an optimal approach to removing carbon dioxide from the air will depend on having an economic incentive for doing so, the key to this being useful materials. Coupled with the only sustainable form of energy that exists, nuclear energy, it seems remotely possible - although by no means certain - that we can reverse the indifference and self delusion that have led to the destruction of the planetary atmosphere. Some of it will involve little steps, like anodes from biomass, and some other large steps, like displacing all dangerous fossil fuel plants with nuclear plants, rendering so called "renewable energy" - which is decidedly not sustainable precisely because of its mass requirements - unnecessary and superfluous.
These ideas of mine are, of course, not popular, but I have convinced myself it would be morally unacceptable, for me personally at least, not to state them.
This little paper made me quite happy, since the issue of anodes has been troubling me for some time, given my fondness for metallurgy and my excitement over the new vistas opened by the Cambridge FFC process, and electrolytic process similar to that of the Hall-Heroult process long in use for aluminum.
I hope and trust that even in the age of Covid, some part of your summer has been pleasant and rewarding.
Susan Collins tweets: she sent a LETTER to the Post Office that she's concerned about their service.
You can't make this stuff up.
Sen. Susan Collins
@SenatorCollins
The USPS continues to be a lifeline amid COVID-19, especially for seniors, veterans, & those in rural areas who rely on mail delivery for essential goods. I sent a letter calling for the USPS to promptly address the delays in mail delivery.
https://collins.senate.gov/newsroom/senator-collins-calls-usps-address-delays-mail-delivery
https://twitter.com/SenatorCollins/status/1294070606544216064
A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV2
The paper to which I'll refer is this one: A mathematical model reveals the influence of population heterogeneity on herd immunity to SARS-CoV-2 (Tom Britton1,*, View ORCID ProfileFrank Ball2, View ORCID ProfilePieter Trapman1, Science 14 Aug 2020: Vol. 369, Issue 6505, pp. 846-849)
The scientific publishing community has made all SARS-Cov-2 papers open sourced. There is no need for me to excerpt all that much of it, but it does note that something that all of here know, even if the ignorant white supremacist in the White House is as clueless on this subject as he is on all others: In diversity is strength.
The paper, however, does not actually refer to ethnic diversity, but rather to diversity in age and activity levels, as well as the diversity in restrictions utilized. This, of course is good news.
A few brief excerpts:
By 1 May 2020, some regions and countries reached high estimates for the population immunity level; for example, 26% of the population was infected (with a large confidence interval) in the metropolitan Stockholm region, as shown by a mathematical model (2). At the same time, population studies in Spain showed that in the second half of May 2020, >10% of the population of Madrid had antibodies for coronavirus disease 2019 (COVID-19) (3). It is debatable whether (classical) herd immunity for COVID-19, which is believed to lie between 50 and 75%, can be achieved without unacceptably high case fatality rates (4–6).
There is then a discussion on the likely impact of vaccination on herd immunity, depending on the efficacy of the vaccine.
A figure from the paper:

The caption:
Shown is a plot of the overall fraction infected over time for the age and activity structured community with R0 = 2.5 for four different preventive levels inserted 15 March (day 30) and lifted 30 June (day 135). The blue, red, yellow, and purple curves correspond to no, light, moderate, and severe preventive measures, respectively.
An interesting read, I think.
Have a nice weekend.
An honorable retraction of a highly cited paper on renewable energy based ammonia synthesis.
When I opened up the issue of Science this morning, I came across this rather - in my opinion - honorable retraction of a six year old paper on the production of ammonia using so called "renewable energy." (Ammonia, the synthesis is critical to the world food supply, is overwhelmingly synthesized using dangerous natural gas, followed by coal, as an energy source.)
The retraction is here: Retraction Stuart Licht1,*, Baochen Cui1, Baohui Wang1, Fang-Fang Li1, Jason Lau2, Shuzhi Liu1 (Science, Vol. 369, Issue 6505, pp. 780 14 Aug 2020)
Many, most, retractions involve some level of fraud, but I don't think this is the case here; the paper may represent an honest mistake, although it does appear that the origins of the mistake were uncovered in other labs.
The full (brief) retraction text:
I have not looked into this paper to any extent, but apparently, according to Retraction Watch, it was highly cited, being in one of the most prestigious journals in the world:
Authors retract highly cited 2014 Science paper
The paper, “Ammonia synthesis by N2 and steam electrolysis in molten hydroxide suspensions of nanoscale Fe2O3,” has been cited 323 times, according to Clarivate Analytics’ Web of Science, earning it a “hot paper” designation. According to a summary of the work, “the protocol points to a way to produce ammonia from purely renewable resources.”
Using the Carbon in Phytoremediation Grasses to Remediate Heavy Metal Brownfields to Make Biofuel.
Like Joe Biden's search for a woman to be his VP candidate - Kamala Harris is clearly outstanding given the group from which she was selected - he current issue of ACS Sustainable Chemistry & Engineering is an embarrassment of riches, at least for me with my particular set of interests in the future of the world I will leave soon enough. The paper among this rich set that I choose to discuss this morning, as I have a little free time to myself having taken the day off, is this one: Valorization of Phytoremediation Byproduct via Synthesis of Biodiesel from Cockspur Grass (Echinochloa crus-galli) Seed (Sungyup Jung, Minyoung Kim, Hyeran Moon, Young-Kwon Park, Jörg Rinklebe, Chang-Jin Park, and Eilhann E. Kwon, ACS Sustainable Chem. Eng. 2020, 8, 31, 11588–11595)
In terms of full disclosure, about 20 years ago I had a semi-serious interest in going into the biodiesel industry, but thankfully thought better of it. I now understand that biodiesel, like most of the so called "renewable energy" strategies that were supposed to address climate change but didn't, aren't and won't, has proved to be destructive to the environment. One still can't say that freely among my fellow political liberals, but facts are facts and facts matter. The primary reason behind this destructive aspect is connected with land use, in particular the destruction of rain forests to use the land for palm oil plantations; one of the larger drivers of climate change besides the criminal habit of mining dangerous fossil fuels and dumping the waste directly into the atmosphere is land use changes.
But as we address the issue of land use - if we address the issue of land use, we should recognize that there is a considerable amount of land that has been functionally destroyed because of contamination with various industrial by products. Some of these by products are organic molecules, notably halogenated organic molecules like PCB's, PBDE's, PFOS, PFOA...ad infinitim but in other cases they are heavy metals.
An area for remediating contamination of land that has received considerable attention is phytoremediation, which depends on plants that concentrate the contaminants in their biomass, thus removing it from the contaminated areas. This is related to the biomagnification of pollutants, like for instance, the volatile neurotoxic coal waste mercury in seafood, and other foodstuffs. Mercury of course is responsible for the madness of hatters that was captured in Alice in Wonderland. (I often speculate to myself that the intellectual decline we are seeing so well represented by Trump Republicans and many other similar types around the world is not related to the wide distribution of neurotoxic heavy metals, in particular mercury, lead and cadmium.)
Anyway, this cool little paper caught my eye, because my primary environmental focus is process intensification, a contention that there should be no such thing as "waste" at all if future generations are to have even a remote shot at cleaning up the mess we left for them with all our wishful thinking, selfishness and generalized bullshit.
From the introduction to the paper:
Biomass collected from phytoremediation sites can be valorized for biofuel production, and this approach also serves as a CO2 mitigation measure.(11) In detail, global energy consumption in 2018 reached to 14 billion tons of oil equivalent,(12) with more than 80% generated from fossil resources.(13) The heavy reliance of energy consumption on fossil resources has become the main contributor of increased atmospheric concentrations of CO2.(14) Nonetheless, substantial efforts have been made over the last few decades to reduce atmospheric CO2 levels.(15,16) Among them, energy production from a carbon neutral resource (i.e., biomass) has been gaining attention because the intrinsic carbon neutralities can balance atmospheric CO2 levels.(17) As biomass is the only carbon-based material among various renewable energies (e.g., photovoltaic, wind, hydroelectricity, tidal, and others), converting biomass into chemicals (including biofuels) based on the concept of biorefinery has also been spotlighted.(18,19)
Very often, far too often for my taste, one sees in the scientific literature the claim that "photovoltaic, wind, hydroelectricity, tidal, and others" are sustainable technologies. They are not. The reason is physics, the extremely low energy to mass ratio of the materials required to make them operative, and the vast amounts of land they are required to consume through destruction, contamination, or simply industrialization. If we are to save the world, in my opinion, these obeisances have to stop.
Anyway...anyway...
It is true that biomass is a potentially sustainable way - even though as practiced now it actually records a higher death toll than Covid-19 - to capture carbon dioxide from the atmosphere, so it is not wise to throw the baby out with the bath water.
Biodiesel, which is currently made on an industrial scale, is composed of "FAME" for Fatty Acid Methyl Esters. All fats are basically triesters (and sometimes diesters or monoesters) of fatty acids, straight carbon chains terminating in a carboxylic acid group, with the trialcohol glycerol. The biodiesel process is to hydrolyze these triesters, and reesterify them with methanol, methanol being a chemical produced in large quantities - on a hundred million ton scale per year - from dangerous natural gas. I believe that methanol, and in particular the symmetric ether made from it, dimethyl ether, DME, is the key to a sustainable future, albeit only if made from the hydrogenation of carbon dioxide not obtained from dangerous natural gas.
More from the authors about their work:
Good stuff, I think.
Some pictures from the paper reflecting the results of their study:

The caption:

The caption:
For the preparation of biodiesel the grass seeds were thermolyzed, that is, treated with heat in the absence of oxygen. The seeds were heated to temperatures of 900°C at a ramp rate of 10°C in a TGA device (thermogravimetric analytical device). This is not highly scaled chemistry: It is effectively microscale. Nonetheless, if the process were to scale, sustainable heat of 900°C is obtainable from nuclear energy in a carbon neutral setting, with such temperatures allowing for process intensification, allowing for the production of, for example, electricity as a side product.
More pictures:

The caption:
In my old age I have come to love thermoanalytical chemistry.
The next graphic shows the interesting fact that some of the fatty acids in this plant's seeds are unsaturated acids. This suggests their use to make other important industrial chemicals besides biodiesel.

The caption:

The caption:
The authors chose to evaluate alcohols other than methanol for esterifying the fatty acids.
When I thought about biodiesel as a kid, I always thought about mixed alcohol waste streams in lieu of methanol. (These choices can have some problematic issues in diesel engines but are perhaps useful in the displacement of dangerous petroleum based home heating oil with biodiesel. Dangerous home heating oil and dangerous diesel fuel are identical chemically, except for the dyes added to home heating oil to prevent its use for tax avoidance diesel fuel.

The caption:
An excerpt from the author's conclusions:
The authors do not much discuss the fate of the metals removed from the soil. Among the other crimes we have committed against all future generations is the depletion of high grade ores of several important elements in the periodic table. It seems to me that much of the world's future energy requirements will thus involve recovery of elements from dilute sources, often the waste piles we so selfishly left. Figures 1 and 2 give a feel for the amount of metals that might be recovered from brownfields.
It's an interesting little paper; I enjoyed thinking about it very much.
I trust you will have a safe and pleasant weekend under the circumstances resulting from the grotesque mismanagement of our safety by the dogma driven fools in the Republican Party. In particular, I hope you will revel in the thought of our superb candidates, Joe and Kamala, in taking the reins and repairing whatever can be repaired after all this willful destruction,.
Steminists.
I was referring to a correction paper in the recent issue of Chemical Reviews and following it around lazily, I came across this interesting and inspiring site for women scientists working at UC Berkeley.
Steminists
What I found particularly interesting is some examples of women who entered the university in non-science majors, and stumbled into science after taking a math class or something of that nature.
For example, here is the graduate student Rachel Woods-Robinson who is a third year graduate student in Applied Physics at UC Berkeley who entered ULCA as a college freshman hoping to major in playing the trombone.
Recently I had a conversation with a young woman who complained that during interviews for her admission to graduate school, people kept telling her that she was a strong candidate because she is a woman of color. She didn't think it was right, felt that she was privileged. (I happen to know that she was also privileged by coming from a wealthy family, but I didn't go there.)
(She shares ethnicity with 50% of our fabulous VP nominee.)
Being an old man - an old white man with a white wife and two white sons - I told her that we all have opportunities based on our backgrounds: My sons for instance got to go to better schools than my wife did - she went to an inner city school - and that was an "unfair" advantage for them.
We in this country are working to smooth out the obstacles before each of us, and if it was a little more difficult for my son to get into an engineering program than it was for some women of color because they are women of color, well, that evens things, doesn't it?
It is not why we have opportunities, I said, but what we do with them when we get them.
Anyway, it strikes me as wonderful that these young STEM students are celebrating being women STEM scientists.
We need more of this, not less of it.
In honor of our wonderful VP candidate...Let's do Frank...
I can hand carry my absentee ballot to the Board of Elections and Circumvent Trump's Destruction...
...of the Post Office.
Concerned about the politicization of even the US Post Office, I emailed my commissioners. Here is the exchange:
You are entitled to deliver your Mail-In Ballot to the Board's Office and you may utilize any secure drop box located throughout the County. The list of drop boxes to place the ballot in will be updated prior to the November Election. Please note that if you and your family elect to hand deliver Mail-In Ballots to the Board, the ballot must be completed prior to arriving at the window. The Staff will have a form to complete to confirm your delivery.
Anthony R. Francioso
Mercer County Board of Elections
From: XXXX <XXXX@gmail.com>
Sent: Saturday, August 8, 2020 12:37 PM
To: boardofelections mercercounty.org <boardofelections@mercercounty.org>; Corrigan, Mary <mcorrigan@mercercounty.org>; Francioso, Anthony <afrancioso@mercercounty.org>
Subject: Questions connected with absentee ballots.
Dear Commissioners:
It has become clear that the US Post Office is under attack in connection with the election, and efforts are being made to politicize it by the officials in the current federal government.
In connection with my personal responsibility to help our democracy survive, I want to be sure my vote will be counted and not trashed or deliberately "lost."
As such, can I and my family request an absentee ballot and hand deliver the filled out ballots directly to the board of elections?
Thanks in advance for your answer.
Best regards,
XXXXX
Such and Such Road.
Some town somewhere, NJ ZZZZZ
This is what I'm going to advise all four Biden voters in my family to do.
Liquid/Liquid Extraction Kinetics for the separation of Americium and Europium.
The paper I'll discuss in this post is this one: Liquid/Liquid Extraction Kinetics of Eu(III) and Am(III) by Extractants Designed for the Industrial Reprocessing of Nuclear Wastes (T. H. Vu, Jean-Pierre Simonin*, A. L. Rollet, R. J. M. Egberink, W. Verboomm AE Enschede,
M. C. Gullo, and A. Casnati, Ind. Eng. Chem. Res. 2020, 59, 30, 13477–13490.)
I have come to consider americium as a critical nuclear fuel if we are ever to get serious about addressing climate change, something about which clearly are not at all serious.
(Solar cells and wind turbines haven't cut it, aren't cutting it and won't cut it. The reason is physics, the extraordinary low energy to mass ratio of these systems which leads to them being purely and totally unsustainable, and in fact, environmentally odious.)
The advantages of americium nuclear fuel include these:
1) The melting point of the metal, while not as low as that of plutonium, is sufficiently low to be accessible for containment for long periods of time. The metal also has a very high liquid range, comparable to that of neptunium and gallium.
2) The critical mass in the fast neutron spectrum (which I regards as a superior spectrum) is much higher than it is for plutonium for the two most common isotopes, Am-241 and Am-243. I discussed these critical masses in this space here: Critical Masses of the Three Accessible Americium Isotopes. The continuous recycling of plutonium will lead to rising critical masses because of the increases in the proportion of Am-243 with respect to Am-241.
3) Over a long period of operation Americium will produce two valuable plutonium isotopes that are critical for denaturing weapons grade plutonium, Pu-238 and Pu-242.
4) Considerable inventories of Americium exist and are readily available owing to the environmentally disastrous fear and ignorance that have prevented the prompt recycling of nuclear fuels.
5) The accumulation of Curium-242, Curium-244, and Plutonium-238 in americium based fuels, besides generating heat, will also generate supplies of the important industrial gas helium, a gas which is rapidly being depleted from mined sources.
6) Over the long term, the decay of Pu-238 will produce important supplies of U-234, which will eliminate, largely, the need for isotopic enrichment of uranium (particularly when conducted in concert with the use of thorium based U-233.)
Besides these advantages, it is possible, but not known to my knowledge, that liquid americium metal will prove to be less corrosive than liquid plutonium. That would simplify things a bit, although in my opinion, the corrosive nature of plutonium is a surmountable problem.
The evolution of an americium based liquid metal fuel over a period of decades will result in an Americium-Plutonium-Uranium alloy. Here is a (computationally derived) ternary phase diagram of this alloy showing liquidus curves:

The caption:
Source: Ogawa, Journal of Alloys and Compounds, 194 (1993) pp. 1-7.
As the fuel evolves, the melting point decreases, and with it, the expectation of sufficiently liquid fuels to allow for in line fuel processing, with the caveat being the effect of fission products.
Europium is a fission product, a relatively minor fission product, but a fission product all the same. It is always to be expected to be present in used nuclear fuels, both from the capture of neutrons in samarium isotopes as well as a direct fission product. Overall, one would expect, qualitatively for europium to relatively depleted because the two natural isotopes Eu-151 and Eu-153, high neutron capture cross sections, at least in the thermal spectrum, as do the two fairly long lived radioactive isotopes and their nuclear isomers, Eu-152 and Eu-154, with half-lives respectively for their low energy isomers, of 13.5 and 8.6 years. Although small quantities of these isotopes probably represent burnable poisons, and may have some utility as such, it may be, and most likely is desirable to remove europium from americium.
This brings me to the paper under discussion. Liquid/liquid extraction (often abbreviated "LLE" ) has been the most common approach to reprocessing used nuclear fuels. I don't necessarily endorse these as being likely to be the best approach, but nobody cares what I think anyway. Almost always, these extractions require agitation and worse, the use of solvents obtained from dangerous fossil fuels. It has occurred to me in recent years, particularly in light of the development of low temperature ionic liquids, that there may be other ways to exploit mass transfer across liquid interfaces, and thus this paper is of potential interest for me as I develop my generally useless thinking.
From the introductory text of the paper:
Various strategies have been developed worldwide for the reprocessing of used fuel. An overview of the main solvent extraction processes(2) (besides Europe) is presented in Table 1. References are indicated in the table that give more details on the policies of the countries in this domain.
Table 1:

The text continues:
Reference aqueous separation process routes have emerged from these in-depth studies. They are depicted in Figure 1.(10,11)
Figure 1:

The caption:
Some descriptive text:
In the other route, the PUREX (plutonium, uranium, reduction, and extraction) process,(14) first implemented in the Manhattan project, is employed for the separation of uranium and plutonium from other fission products by using tributyl phosphate (TBP) as the extractant. The COEX process is a modified version of PUREX. Then, the DIAMEX (DIAMide EXtraction) process developed at CEA (Commissariat à l’Energie Atomique) in France may be used. It consists of the co-extraction of trivalent minor actinides [MA’s, mainly composed of americium(III) and curium(III)] and lanthanides (Ln’s) from a PUREX raffinate by employing a malondiamide extractant. Although they constitute less than 0.1% of the initial spent fuel mass, the MA’s (especially neptunium, americium, and curium) will be the main contributors to the radiotoxicity (and heat generation) after a three-century storage of high-level radioactive liquid waste (obtained after the PUREX stage).
The authors here are referring to the "storage" of so called "nuclear waste." By contrast, I speak of the recovery of nuclear resources. They are also speaking of "once through" thermal fuel, largely, and not continuously recycled actinides.
These caveats aside - most of humanity has been trained to think in this way, of waste rather than resources and this is clearly a fatal way to think, fatal to the future.
In a continuous actinide recycling program, some calculations show that it is possible to obtain americium alone in concentrations of close to 1.5% (cf. Ref: Nuclear Reactor Physics, William E. Stacy, Wiley and Sons 2001. pg.234). In a world in which we we did not use any dangerous fossil fuels, where we let our rivers run free, where we did not convert our wilderness into industrial parks for wind turbines, destroy rain forests for biofuels, generate millions of tons of toxic electronic waste for solar cells, we would need, in order to produce 600 exajoules of energy per year that we use as of recent times, we would require the fission of about 7,500 tons of plutonium (or other actinides) per year. This implies about 100 MT of americium would be available per year, a significant quantity of potential industrial importance. There may not be a lot of americium, but for the reasons given above, it may prove a useful fuel.
The authors use a device known as "rotating membrane cell" which is pictured here:

The caption:
The technique is closely related to solvent extraction techniques used in the industry, with several extractants in a commercially available mixture of dodecane isomers known as "TPH" containing a small amount of n-octanol, whereupon the solvent is known as "TPH-O." The extractants utilized in these solutions are shown in the following figure:

The caption:
The membranes employed are commercially available, one being a hydrophilic membrane, the other hydrophobic:
There is considerable discussion in the paper of the technique, and there is not time to describe all of it in detail. However the separation efficiency and speed are significant.
The last figure in the paper shows distribution coefficients for one system evaluated:

The caption:
The authors conclude, noting that the purpose of their work is to provide input for further modeling and development of new systems.
The kinetic data obtained in this work will be used as input parameters in simulation codes (such as, e.g., PAREX, developed at CEA(64,65)) for a modeling of separation processes carried out in extractors (e.g., centrifugal) that operate with a short contact time between the phases.
The experimental results, obtained with TODGA and the two aqueous stripping ligands, show that faster transfer kinetics are associated with higher partitioning for Am(III) over Eu(III). This favorable outcome bodes well for future efficient actinide/lanthanide separation in the nuclear reprocessing industry.
My personal feeling is that we need to move beyond nitric acid dissolution of used nuclear fuel, which is a feature of the chemistry herein.
I believe the cleaner option for future nuclear fuel reprocessing is to conduct some of it in line using techniques including but not limited to distillation. There are also possibilities, some of which were briefly explored in the 1950's, to utilize liquid/liquid extractions in line using inorganic liquids.
However, it may be that liquid/liquid interfaces may be important at some point in future techniques. We need to take a deeper look at molten salt based separations, included but not limited to organic ionic liquids. Much attention is being paid to these substances. Finally a driving force for both separations and dissolution may not need to involve mechanical forces. Electrochemical techniques, including those involving liquid membranes are worthy of consideration.
There are really a wealth of options for the treatment of nuclear fuels and the recovery and use of the radioactive and non-radioactive materials therein. These materials are probably, in my view, the best shot we have to save the world.
I hope your weekend was pleasant as much as it was safe.
Susan Collins.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512