NNadir
NNadir's JournalI like this year's AAAS tee shirt.
Every year at Christmas, Santa slims down and slithers through the wires and circuits of my computer to renew my AAAS membership and subscription to Science.
Later he sends a tee shirt by US Mail.
I like this year's teeshirt:

If it's hard to read the letters on the helix:
"Diversity, it's in our DNA."
It's kind of a scientific pun, if you must know.
A Round Robin Evaluation of the Radiation Stability of Actinide Extraction Reagents for Nuclear Fuel Recovery.
The paper I'll discuss in this post is this one: Interinstitutional Study of the New EURO-GANEX Process Resistance by Gamma Irradiation Test Loops Ivan Sanchez-Garcia, Xavier Heres, Dean. R. Peterman, Hitos Galan, Sylvain Costenoble, Sylvain Broussard, Johann Sinot, Travis S. Grimes, Kash Reid Anderson, Santa Jansone-Popova, Maria Chiara Gullo, Alessandro Casnati, Andreas Wilden, and Andreas Geist Industrial & Engineering Chemistry Research 2023 62 (47), 20326-20339.
Recently in this space, I discussed in this space the ability of a biological protein, lanmodulin, to complex the higher anthropogenic actinide elements americium and curium:
A Bacterial Protein Can Transport Heavy Highly Radioactive Actinides.
From the text of the paper discussed therein, I quoted this excerpt:
Note: I have substituted "approx." for the traditional symbol in the original text, which does not yet show up in DU4.
As I favor the continuous recycling of used nuclear fuels to recover the valuable resources therein, I hope that we will see more - a lot more - use of mixed uranium-plutonium fuels, and thus larger quantities per ton of americium and curium.
The heaviest actinide nuclide that does not have a critical mass occurs naturally, 238U, which makes up the bulk of the billion ton quantities of uranium naturally occurring in the Earth's crust and oceans, even without respect to the planetary mantle, which does cycle through the crust. 238U can be and is transmuted into heavier actinides all of which do have critical masses, including all of the isotopes of neptunium, plutonium, americium and curium, all of which can be isolated from used nuclear fuels, and thus can be utilized to produce clean primary energy.
I have referred to the critical masses of neptunium here: Experimental Determination of the Bare Sphere Critical Mass of Neptunium-237. (Unfortunately the graphics in that post have expired, and I will not have time to restore them.)
I have referred to the critical masses of americium isotopes here:
Critical Masses of the Three Accessible Americium Isotopes. (The graphics in this post are intact.)
Since both elements have critical masses, they are potential sources of clean primary energy. Nonetheless, they are still thought of as "nuclear waste" with the unfortunate use of this evocative but rather stupid and misleading arbitrary locution rather resistant to meaningful address, and thus discussed in terms of their radiotoxicity and heat loads.
Before proceeding further, let me state that I am not a solvent extractant kind of guy, even though almost all of the isolation of plutonium to make MOX fuels (chiefly in France and to a lesser extent, Britain) has relied on an industrial scale solvent extraction process, PUREX (Plutonium/Uranium Reductive EXtraction). The process discussed in this paper is called GANEX (Group ActiNide EXtraction). Both of these involve extraction into water immiscible solvents using complexing agents. In PUREX this solvent has been historically, on an industrial scale, kerosene. Dodecane, another product of dangerous fossil fuels is often considered as an extraction solvent, although in theory this can be made by the deoxygenation of the fatty acid lauric acid from biological matrices.
As opposed to a solvent extraction kind of guy, I am a pyroprocessing kind of guy, in favor of using molten salt chemistry and related chemistry, and or processes known as fluoride volatility, voloxidation, electrorefining and other techniques to separate the 45 or so elements in the periodic table formed in nuclear fuels during fission based operations. These processes require heat, something that fresh used nuclear fuel generates quite readily, or in the case of older used nuclear fuels, can be generated by operating nuclear reactors.
This said, I can see limited applications in which solvent extraction approaches may have limited use, particularly, if desired, the separation of americium and curium from their lanthanide congeners and other lanthanides.
It is the radioactive lanthanides in the presence of actinides that are the focus of the paper under discussion.
One of the many advantages of radiation as a tool is its ability to break chemical bonds. Photosynthesis, for example, utilizes visible light radiation to break water's chemical bonds. Some bonds require more energy to break than those in water do. This is particularly important for the class of ubitiquous pollutants known as "PFAS" which contain multiple carbon fluorine bonds, among the strongest chemical bonds known. The energy to cleave them lies not in the visible spectrum, but in the far UV spectrum; the energy required must have wavelengths on the order of 220 nm or shorter to break these bonds, going into the x-ray and gamma spectrum. When I think of ways to address the very serious issue of PFAS contamination, the use of radioactive fission fragments is very high on my intellectual radar.
In aged used nuclear fuel, some of the lanthanides are no longer highly radioactive: Lanthanum, praseodymium, neodymium and gadolinium can be isolated from these fuels with radioactivity levels consistent with or even less (in the cases of lanthanum and gadolinium) radioactive than the lanthanides found in natural ores. Cerium in fresh used nuclear fuels has a relatively short lived radioisotope 144Ce, with a half life of 284 days, that will require about a decade of cooling for the bulk cerium to consist of stable or metastable isotopes. (With respect to metastability, some natural isotopes of cerium are thought to be slightly radioactive, but with half-lives comparable to the age of the universe.) Europium, and samarium, by contrast, have radioisotopes found in used nuclear fuel that have half-lives on the order of years and decades. In theory, although probably not in practice, both europium and samarium, which have characterized +2 oxidation states, could be isolated by redox procedures, but this would be messy. In nuclear fuels they are almost certainly in their more stable +3 oxidation state and are probably better addressed as such. Promethium does not exist on Earth except, by suspicion but not - to my knowledge - direct observation, in vanishingly small amounts uranium ores from spontaneous fission. All of its isotopes are radioactive, although only one is likely to be in used nuclear fuels 147Pm, with a half-life of 2.62 years. Happily the radioactive lanthanide isotopes of samarium, europium and promethium all have fairly high neutron capture cross sections - they could be used as control rods - and their concentrations are thus suppressed, but they do accumulate and are found in used nuclear fuels. The accumulation of samarium isotopes is responsible for the shut down of used nuclear fuel rods before the fissionable isotopes are consumed.
Solvent extraction relies on the use of molecular complexants, these are organic molecules, and as such, in radiation fields are subject to chemical bond cleavage. That is the focus of this paper on the GANEX process, a concern being the lanthanides that are radioactive.. Note, as well, that all of the actinides are radioactive, in particular curium and americium.
From the introductory text of the paper:
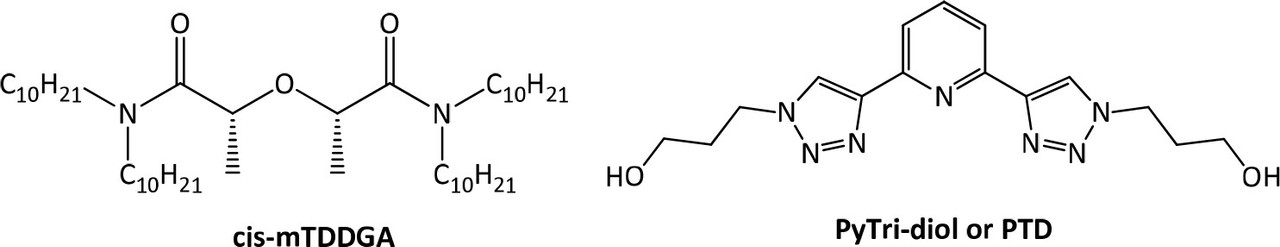
Within the homogeneous strategy, which addresses the recycling of U and the transuranic elements (TRU = Np, Pu, Am, Cm) contained within a single fuel type and distributed homogeneously throughout the reactor core, GANEX (Group ActiNide EXtraction) is the most promising process to recover all of them. (9) In the GANEX concept, bulk uranium is removed in a first cycle, followed by the coextraction of all actinides in a second cycle. Three options exist for this second cycle, the CEA-GANEX, EURO-GANEX, and CHALMEX processes. (4,8) The EURO-GANEX is one of the most promising options to achieve the desired goals (10) and is based on the coextraction of all actinides using a mixture of N,N,N?,N?-tetraoctyl-diglycolamide (TODGA) (11,12) and N,N?-dimethyl-N,N?-dioctyl-2-hexylethoxy-malonamide (DMDOHEMA) (13) extractants. TODGA exhibits high affinity for actinides and lanthanides, but the addition of DMDOHEMA is essential to avoid precipitation caused by the high Pu concentration in the organic phase. (10,14) After the coextraction of An and Ln into the organic phase, a separation between both can be obtained by selective stripping of the actinides as a group, using a mixture of 2,6-bis(5,6-di(sulfophenyl)-1,2,4-triazin-3-yl)-pyridine (SO3-Ph-BTP) (15) and acetohydroxamic acid (AHA). (16) The EURO-GANEX process was tested in an irradiation loop (17) and successfully demonstrated with high Pu content using genuine nuclear fuel solution in centrifugal contactors for the first time, (18) obtaining excellent results. However, EURO-GANEX also has some drawbacks: One of them is that the combination of two extractants in the organic phase (TODGA and DMDOHEMA) complicates solvent management; another one is that the sulfonated BTP reagent employed in the aqueous phase does not accomplish the “CHON principle”, (19) leading to troublesome sulfur-containing byproducts. Therefore, the process needs to be further optimized to meet the criteria mentioned above and to simplify the process as much as possible...
...In order to replace SO3-Ph-BTP, a novel molecule which meets the CHON principle, 2,6-bis[1-(propan-1-ol)-1,2,3-triazol-4-yl)]pyridine (PyTri-Diol, or PTD) is proposed (Figure 1, right). This agent was found to have high actinide selectivity and radiochemical stability (26?31) and could be suitable for the application as a stripping agent not only in GANEX process but also in i-SANEX (Innovative Selective Actinide Extraction) process involved in the heterogeneous strategy of actinides recycling. (28,31,32)
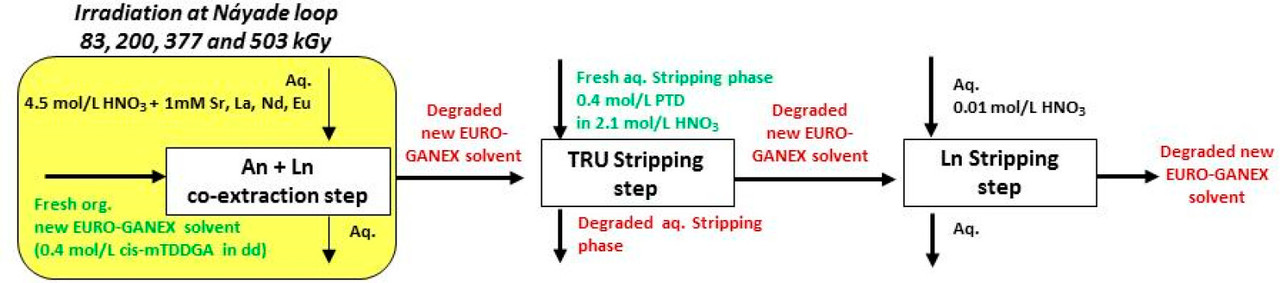
These improvements made to the EURO-GANEX system have resulted in the emergence of the so-called New EURO-GANEX process, with cis-mTDDGA/dodecane (dd) in the organic phase and PTD in the selective TRU back-extraction aqueous solution (Figure 1). As in the EURO-GANEX process, the cis-mTDDGA solvent is useful to extract actinides and lanthanides from an acidic GANEX first cycle raffinate. After this coextraction step, the TRU elements are selectively stripped into the PTD aqueous solution, while cis-mTDDGA maintained rare earth elements in the organic phase...
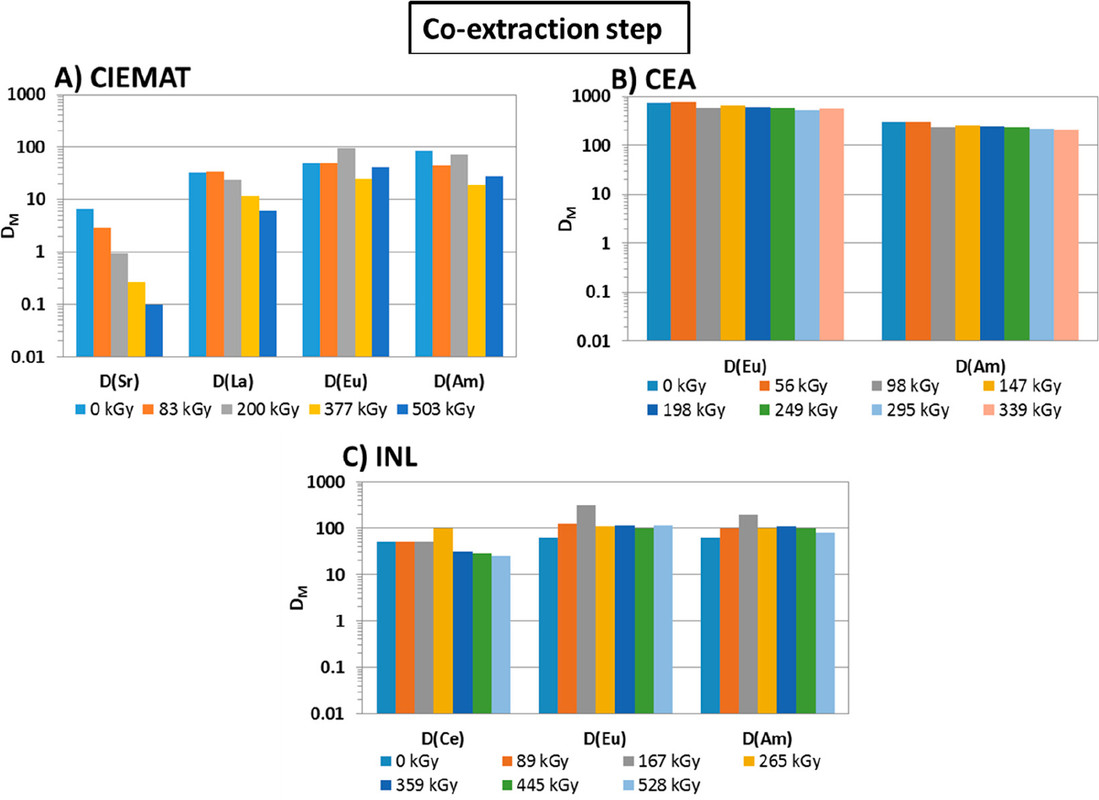
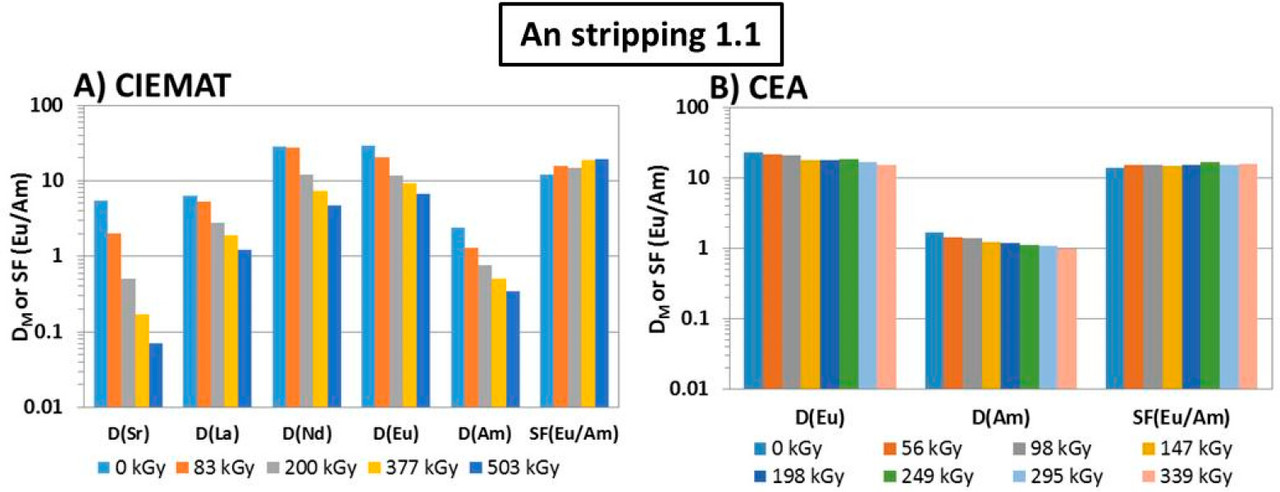
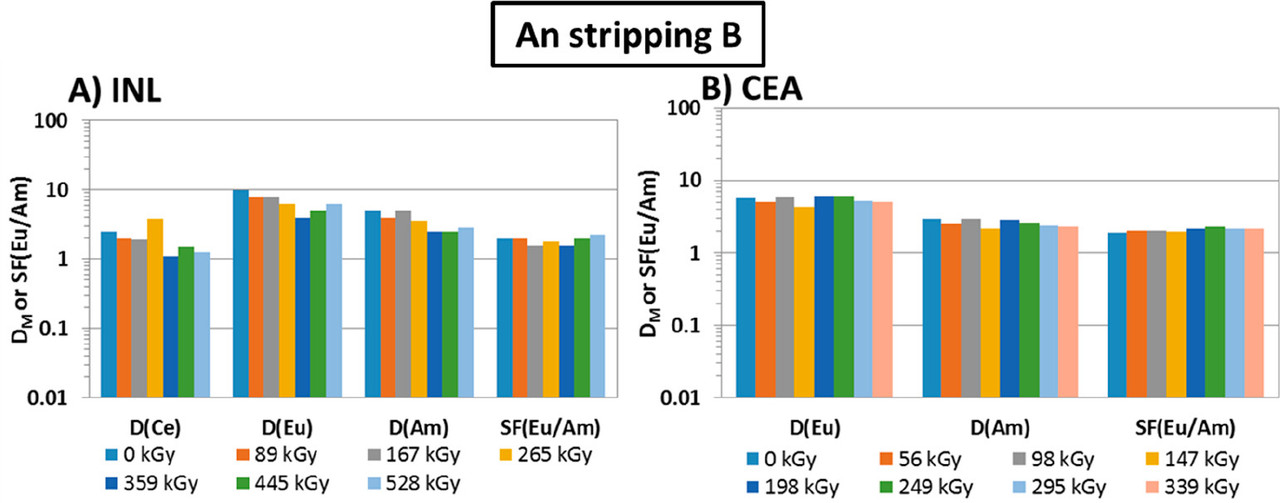
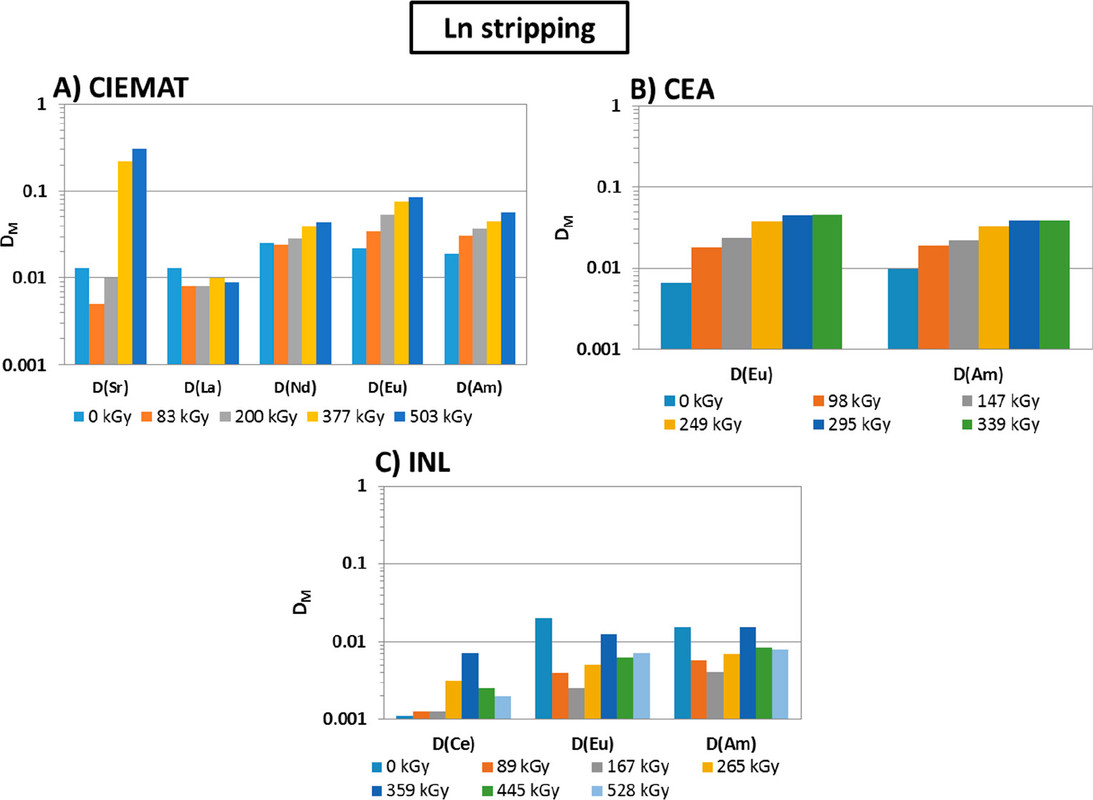
...this work is involved as part of an interinstitutional collaboration between CEA (Commissariat à l’Énergie Atomique et aux Énergies Alternatives) in France, INL (Idaho National Laboratory) in the United States, and CIEMAT (Centro de Investigaciones Energéticas, Medioambientales y Tecnológicas) in Spain. For this collaboration, each research center employed its irradiation loop device to evaluate the resistance of the New EURO-GANEX process to gamma radiolytic degradation using similar conditions and same solutions...
The solvents in this system were subject to radiation exposure using real nuclear fuel solutions as well as radiation generating loops.
Figure 1 from the text:
The caption:
The caption:
Fig
The caption:
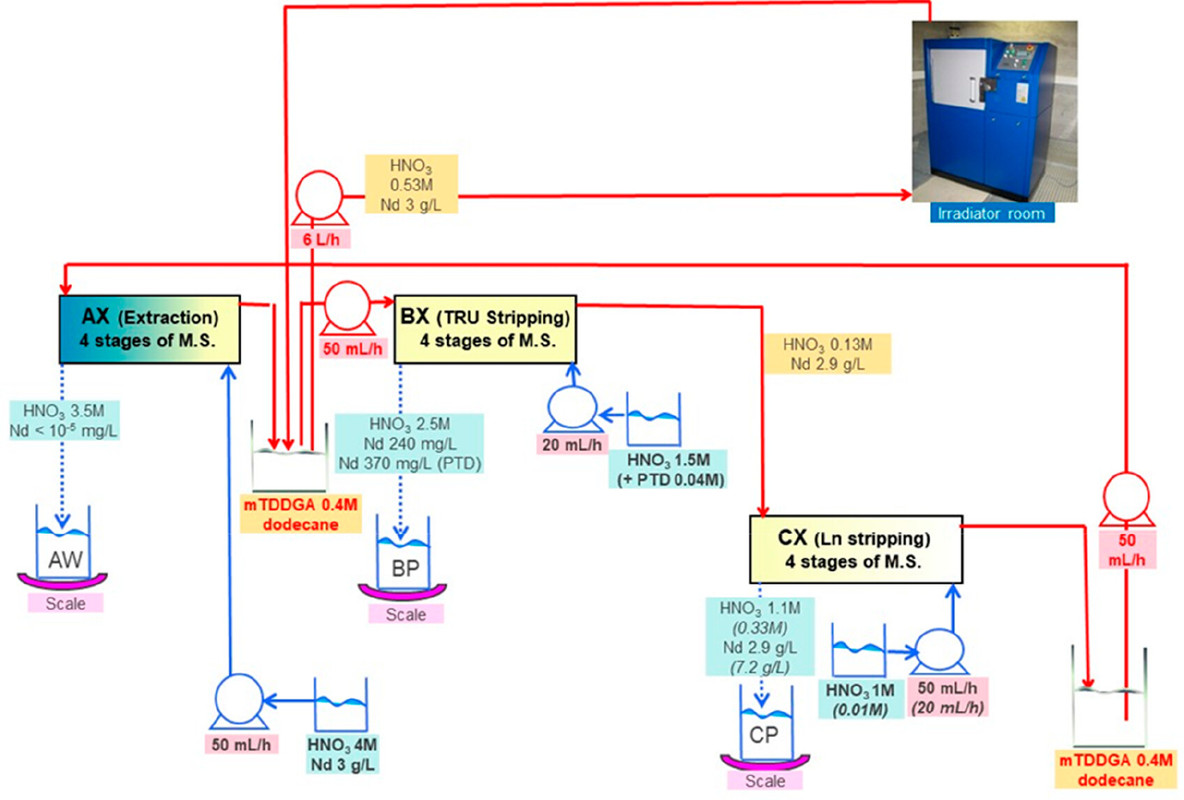
Figure 3. Flowsheet of the loop test following the CX hydrodynamic problem (italics indicate initial value). The CX outflow solvent samples for batch studies were taken after 56, 98, 147, 198, 249, 295, and 339 kGy (M.S. = mixer-settler).

The caption:
The caption:
The caption:
The caption:
The caption:
The full paper gives structures of some of the degradants found.
The international team of authors discuss, in their conclusions, some of the issues found in the GANEX systems evaluated, and note, with minor differences, the similarity of their results.
From my personal perspective, the situation might be improved by removing neptunium and plutonium (along with uranium) as volatile fluorides before undertaking a GANEX procedure to isolate americium and curium, but nobody cares what I think.
I trust you'll have enjoyable holidays.
Weird Justification
This post of mine, More Christmas Chemistry: Key Odorants of Frankincense, cis and trans Olibanic Acids on my computer at least , seems to have weird justification, with space on the right.
I've seen this in other posts, sometimes to the extreme of making posts unreadable, as well, both on my android phone and on my Windows 11 computer. It seems to come and go at random, but is happily a relatively rare occurrence.
More Christmas Chemistry: Key Odorants of Frankincense, cis and trans Olibanic Acids
Earlier today I posted on the chemistry of Myrrh: Christmas Chemistry: Synthesis of an Essential Oil from Myrrh
To repeat what I said in that post:
That post was based on the recent ACS "Molecule of the Week;" this one is based on the previous week's "Molecule of the Week."
Olibanic acids
Excerpts:
Surprisingly, the main odor ingredients in frankincense were only recently discovered. In the 2016 seminal article on this discovery, Nicolas Baldovini and collaborators at the University of Nice Sophia Antipolis (France), Albert Vieille SA (Vallauris, France), and the University of Turin (Italy) reported the isolation and characterization of ( + )-cis- and ( + )-trans-2-octylcyclopropanecarboxylic acids, which they called olibanic acids after frankincense’s alternative name. The 3-D image above represents the more fragrant cis isomer.
The olibanic acids constitute only ?0.2 wt% of the oil derived from frankincense; but the authors’ olfactory tests showed that they are responsible for the aroma of the resin, which they describe as the “smell of old churches”. The researchers synthesized the two (+)-diastereomers as well as their (–)-counterparts, which do not exist in frankincense. The synthetic (+)-diastereomers are identical with the natural compounds that were extracted from B. carterii and B. frereana, species that grow in mountainous regions on both sides of the Gulf of Aden and the Red Sea...
The structure of these stereoisomeric acids is somewhat unusual for a natural product: They have highly strained cyclopropyl rings.
The chemistry, structure and synthesis of these molecules is described here:
C. Cerutti-Delasalle, M. Mehiri, C. Cagliero, P. Rubiolo, C. Bicchi, U. J. Meierhenrich, N. Baldovini,, The (+)-cis- and (+)-trans-Olibanic Acids: Key Odorants of Frankincense Angew. Chem. Int. Ed. 2016, 55, 13719.
A scheme for synthesis is shown in that paper.
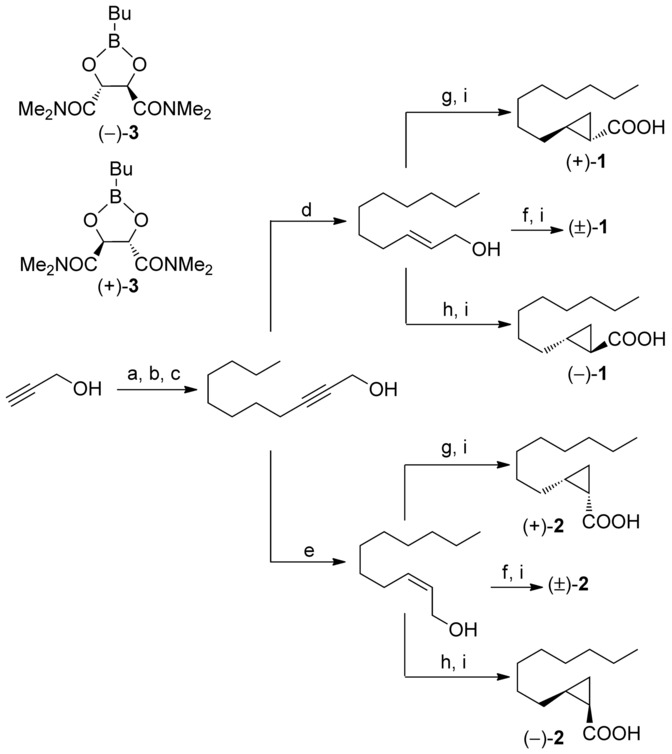
The four diastereomers are on the right.
The caption:
Reagents and conditions: a) 3,4-dihydropyrane, Amberlyst® 15, Petroleum ether, RT, 7 h, 89?%; b) NaH, DMSO (4 equiv), THF, RT, 15 h, then 1-bromooctane, RT, 29 h, 82?%; c) Amberlyst® 15, MeOH, 45?°C, 40 h, 84?%; d) LiAlH4, THF, reflux 2 h, 64?%; e) H2, P2-Ni, 1,2-ethylenediamine, MeOH, RT, 17 h, 87?%; f) Et2Zn, CH2I2, n-hexane, ?35?°C?RT, 13 h; g)
Some text from the paper:
To the best of our knowledge, natural 2-alkylcyclopropane-1-carboxylic acids are scarce: trans- and cis-2-pentylcyclopropane-1-carboxylic acids (4 and 5) are trace constituents of patchouli EO9 and (1S, 2R)-5 has been detected in Mentha gracilis EO (Figure 3)...
...It is surprising that 1 and 2 remained unidentified up to now, while a detailed survey of the litterature shows that many phytochemical studies focused extensively on the volatile fraction of frankincense.14 When considering the main species used in incense burners (i.e. Boswellia carterii, B. sacra, B. papyrifera, and B. frereana) the major volatiles are generally either classical monoterpenes (?-pinene, ?-thujene, limonene) or octan-1-ol and its acetate.14 Interestingly, some old references noticed that both of these types share some common olfactory properties,4b thus suggesting that they may contain common odorants...
Again, I wish you the happiest of holiday seasons consistent with your cultural preferences near or at the winter solstice, and the happiest New Year which will include the reelection of Joe Biden to be President of the United States.
Do you ever visit relatives graves?
This past weekend, my wife was compelled to follow her sister to her parents' graves for Christmas decorations. Her parents were cremated, but the ashes were placed in my father's-in-law parents grave.
After my mother died, I would go to my mother's grave with my father for his benefit, not mine. After he died, I would visit his and my mother's grave with my stepmother as well as the grave of her first husband near by, for her benefit not mine.
I never got the point.
Greenwood Cemetery in Brooklyn has some charm for history buffs, and perhaps students of sculpture: My in laws are buried near the Grave of New York's Boss Tweed, which is sort of mildly interesting although I have better things to do than to contemplate that particular corrupt politician.
But I don't get it, really.
I can remember my parents, and my wife's parents without standing in the rain and cold with a bunch of flowers. I mean, they're dead, and what's in those tiny plots couldn't possibly capture what they were.
I expect now, given the preferences of my sisters-in-law, and out of deference to my wife, I'll probably drop by Boss Tweed again, but I just don't get it and won't get it.
Christmas Chemistry: Synthesis of an Essential Oil from Myrrh
It's probably not entirely Kosher for an atheist to comment on Christian Scriptures, but as a cultural affectation, based on my Christian upbringing and my long participation in American consumer culture, I will anyway.
If there are responses to this post, they are likely to contain clips from Monty Python's Life of Brian.
From the "Molecule of the Week" from the ACS, Furanoeudesma-1,3-diene, the active component from the resinous oil Myrrh which was reported as a Christmas gift from the "Three Wise Men."
Furanoeudesma-1,3-diene
(The world could use three wise men now, but we only have one, Joe Biden.)
From the text, the link, not the Bible:
Myrrh resin and the oil derived from it have been used for millennia as a fragrance and medicine. Along with frankincense1, it is familiar as a gift offered to the Christ child by the Magi in the Christian gospel.
In 1983, Carl Heinz Brieskorn* and Pia Noble at the University of Würzburg (Germany) identified furanoeudesma-1,3-diene and two other aroma compounds in the essential oil of myrrh. Thirteen years later, Jose R. Pedro and co-workers at the University of Valencia (Burjassot, Spain) described the stereoselective synthesis of furanoeudesma-1,3-diene from the drug santonin2. The synthetic product was identical to furanoeudesma-1,3-diene obtained from C. myrrha...
I've called up the 1983 paper to enjoy how far we've come since then with structure elucidation. (It was fine work for its time.)
The synthesis from a starting material chemically derived from the antiparasitic drug Santonin was described here:
Stereoselective Synthesis of 8,12-Furanoeudesmanes from Santonin. Absolute Stereochemistry of Natural Furanoeudesma-1,3-diene and Tubipofurane Gonzalo Blay, Luz Cardona, Begoña García, José R. Pedro, and Juan J. Sánchez The Journal of Organic Chemistry 1996 61 (11), 3815-3819.
The synthetic scheme:
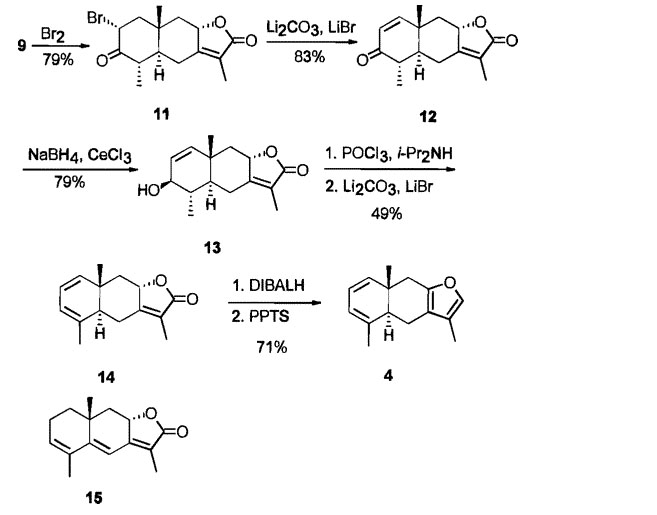
The compound is reportedly a pain killer and has been been demonstrated to bind to opioid receptors. Whether it is, as many such binding compounds are, is not for me to say.
The Christmas season is addictive however. I celebrate it every year in the traditional consumer oriented way.
I wish you a Merry Christmas or holiday of your cultural choice and a happy, healthy and prosperous New Year in which we can celebrate the reelection of Joe Biden.
From the Writer of The Simpsons: Nuclear Power and the Simpsons.
Let me start here: Knowing as I do that The Simpsons is involved with the mockery of the nuclear industry - which I emphatically support - I have never watched a full episode of the show.
I was thus surprised to see this article in my Nuclear News Feed: Nuclear power and The Simpsons
(Registration Required; I am, of course, registered.)
The news feed email has this note:
Some excerpts from the full article:
My own interest in fission started back in Columbia, Mo., in the 1970s, when I participated in a fifth-grade classroom debate about nuclear power. Our teacher assigned me to the “pro” side and gave us a few days to prepare. I met with a neighbor down the street—Marc De Chazal, a chemical engineering professor who helped start the nuclear engineering program at the University of Missouri. He was incredibly generous with his time. I recall furiously scribbling notes for more than an hour.
Can you imagine being the kid who had the “con” side of the debate, armed only with a paragraph from the World Book Encyclopedia? He walked into a fusillade of bullet points and data provided by Professor De Chazal, who’d helped build the highest-power university research reactor in the United States. My opponent never knew what hit him. I remember him looking dazed and stammering, “But . . . but . . . there are concerns. Concerns!...”
...So, yes, DOE-NE, we might have taken some liberties.
However, in an episode I wrote in season 33, we mounted something of a defense of the industry. In “Portrait of a Lackey on Fire,” a new business has come to town—a “fast fashion” company—and Mr. Burns says enviously, “Fast fashion is far more toxic than nuclear power.” Mr. Smithers: “It’s . . . worse?” Mr. Burns: “Nuclear energy gives people warmth and light. This guy is profiting off a product nobody needs: a constant stream of brand-new skinnied jeans and be-cropped tops.” We originally had a longer speech about the clean energy benefits of nuclear power but had to cut it for time. One day perhaps that defense will make it onto the show.
I wish I could have sent that Burns scene to Professor De Chazal. I’m sure that his passion and commitment to nuclear energy has inspired many people in more direct ways, but I like to think that he would have gotten a kick out of the idea that his tutelage of a 10-year-old impacted a Simpsons episode 50 years later...
I was surprised, particularly coming from someone who has done so much to ridicule the industry that is the last, best hope of Earth.
A Bacterial Protein Can Transport Heavy Highly Radioactive Actinides.
The paper I'll discuss in this post is this one: Impact of a Biological Chelator, Lanmodulin, on Minor Actinide Aqueous Speciation and Transport in the Environment Gauthier J.-P. Deblonde, Keith Morrison, Joseph A. Mattocks, Joseph A. Cotruvo Jr., Mavrik Zavarin, and Annie B. Kersting Environmental Science & Technology 2023 57 (49), 20830-20843.
In this space, I previously discussed, at some length - considerable length actually - on a paper written by two of the authors of the paper just cited, Mavrik Zavarin and Annie B. Kersting.. Actually I covered a lot of papers in that post which was fascinating to write, but probably challenging and/or impossible to read.
The post is here: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels.
I fessed up about it right out of the box about the desultory nature of that post, but irrespective of my carrying on about it (and indeed the current paper under discussion), Drs Kersting and Zavarin are outstanding, excellent scientists in my opinion, having had exposure to their work.
Here's what I wrote back then:
One of the papers I'll discuss in this post is this one: Plutonium Desorption from Nuclear Melt Glass-Derived Colloids and Implications for Migration at the Nevada National Security Site, USA (Claudia Joseph, Enrica Balboni, Teresa Baumer, Kerri Treinen, Annie B. Kersting, and Mavrik Zavarin Environmental Science & Technology 2019 53 (21), 12238-12246)
The reference to Dunning, Tunnels and Strawmen was a reference to the inspiration for the post was a moron of my acquaintance who felt it necessary to let me know that a tunnel with some old mildly radioactive rail cars had collapsed on the Hanford Nuclear Site and thus my advocacy of nuclear energy was unworthy. There was a lot of pissing and moaning and whining in follow up interactions with the insufferable idiot, and eventually I put the idiot on my ignore list where he/she/they/it/them happily resides to this day.
"All we are saying, is give peace a chance."
One of the things about antinukes, including "I'm not an antinuke" antinukes, is that they don't know shit from shinola when it comes to nuclear issues, although this doesn't stop them from commenting on them, usually in an insipid fashion.
By the way, your Government spent huge amounts of money, and generated many tons of dangerous fossil fuel waste - diesel exhaust mainly - to address the collapsed tunnel, which saved zero lives, because in reality zero lives were really at risk from radiation, although lives may be at risk from diesel exhaust, because the paranoia of people like the tiresome fool on my ignore list carries way more weight than it should or would in a rational world.
Anyway, to turn to the Dr. Kersting's and Dr. Zavarin's et al paper currently under discussion, it refers to the protein binding of two important (and in my view precious) actinide transuranium elements produced in nuclear power plants, curium and americium.
From the introduction:
Metalloproteins could affect the migration of certain radionuclides as is demonstrated here with lanmodulin and trivalent actinides.
Introduction
In recent years, there has been a renewed interest in using nuclear energy for electricity production (1) due to its low greenhouse gas emissions, small land footprint, and nonintermittent operation. There are currently 412 commercial nuclear fission reactors in operation around the world, (2) 58 are under construction, and several countries have plans to further expand or update their fleet of nuclear fission power plants. There are currently 93 reactors in operation in the United States, including a new 1100 megawatt reactor at Plant Vogtle (Georgia) that entered commercial operation on July 31st, 2023. While fission of uranium-only fuels or mixed uranium–plutonium oxide fuels currently represents one of the means of energy production with the lowest carbon and land footprint, it nonetheless produces long-lived, radioactive waste comprised of fission product, plutonium (Pu), and minor actinide (e.g., neptunium (Np), americium (Am), and curium (Cm)) isotopes that must be managed appropriately. A typical uranium-only civilian nuclear spent fuel, cooled for 5 years, still contains 13–18 kg lanthanide fission products, 10–13 kg Pu, 0.5–0.8 kg Np, ?0.6 kg Am, and 0.03–0.12 kg Cm per metric ton of fuel. (3) For mixed uranium–plutonium oxide fuels, these numbers are 12–17 kg lanthanide fission products, ?30 kg Pu, ?2 kg Np, ?2 kg Am, and ?0.4 kg Cm per metric ton of fuel. The International Atomic Energy Agency estimates (4) that between 1954 and 2016, the equivalent of 390,000 t of U+Pu metal has been discharged from nuclear reactors worldwide. By the end of 2016, about 137,000 t had been reprocessed and 263,000 t remained in temporary storage. Disposal in deep underground repositories is largely seen as the safest option for the long-term management of spent fuels and nuclear waste, whether or not they have undergone reprocessing (e.g., PUREX process (5)). These repositories are meant to isolate the nuclear materials for thousands of years─the time scale required so that the radioactivity returns to a level similar to that of naturally radioactive ores. While the waste packages are designed to initially isolate the radioactive materials when they are the most radioactive, those engineered barriers will ultimately fail due to underground mechanical forces and natural alteration processes. In this context, contact between radionuclides and the underground natural environment (e.g., minerals, groundwater, metal chelators, as well as microorganisms) is expected to take place, and thus, studying the chemistry of radioelements under environmentally relevant conditions is critical to assess the long-term behavior of nuclear waste. (6?10)
My enormous respect for the authors aside, let me comment that I do not agree, in any fashion, with burying any components of used nuclear fuel in "deep underground repositories." I have convinced myself that all of the components of used nuclear fuel are valuable resources, more or less essential for human (and in fact, planetary) survival.
The figures for the actinide components of the fuel are worthy of consideration. The marvelous Vogtle reactors recently completed in Georgia will each be loaded with 82 (metric) tons of enriched uranium oxide. When this fuel is removed from the reactor after use, following the figures above, this suggests, for the upper limits, (first pass without initial plutonium (MOX) fuel) the fuel will contain a little over 1 ton of plutonium, 65 kg of neptunium, 50 kg of americium, and about 10 kg of curium. If we take, as an approximate figure, the fission of an actinide to be roughly equivalent to that of plutonium, 80 trillion Joules per kg, and the US EIA's figure for an average American annual energy demand, determined from the 100 Quads reported by the EIA (106 EJ) for a population 2022 of 333,000,000 million people, giving a per capita demand of 311 GJ/person, the energy content of the combined transuranium actinides in the Vogtle used nuclear fuel (after 3 years of operation) would be enough to provide all of the annual energy demand of around 320,000 people. Note too, that all of the uranium in the used nuclear fuel has the potential to be transmuted into transuranium nuclides.
If we assume that 95% of the 263,000 metric tons of used unprocessed nuclear fuel is unreacted uranium, which can be converted to plutonium and other transuranium nuclides, the energy content of used nuclear fuel is enough to supply all of the world's energy demands (at the 2022 figure of 634 EJ of energy) is enough to fuel the entire world for about 30 years.
Maybe we should think twice before burying the stuff.
Anyway.
It appears that certain ubitiquous bacteria, Methylorubrum extorquens generates a protein, lanmodulin, that has exceptional ability to complex actinides in the +3 oxidation state, having even more affinity for the actinides than the already high affinity for the related lanthanide congeners:
Lanmodulin is a relatively small protein, just 130 amino acids in length according to Uniprot, with the following sequence:
MAFRLSSAVLLAALVAAPAYAAPTTTTKVDIAAFDPDKDGTIDLKEALAAGSAAFDKLDPDKDGTLDAKELKGRVSEADLKKLDPDNDGTLDKKEYLAAVEAQFKAANPDNDGTIDARELASPAGSALVNLIR.
The metal binding sequences are shown here:
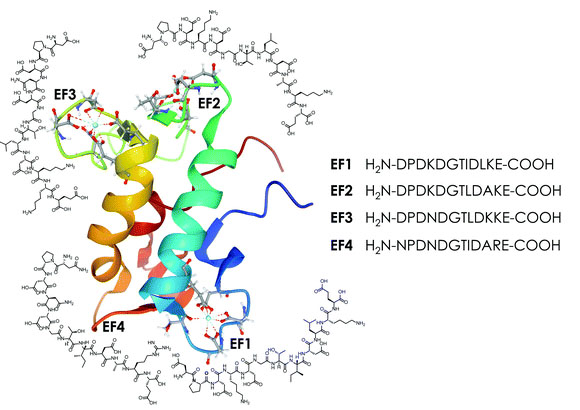
The caption:
Sophie M. Gutenthaler, Satoru Tsushima, Robin Steudtner, Manuel Gailer, Anja Hoffmann-Röder, Björn Drobot and Lena J. Daumann Lanmodulin peptides – unravelling the binding of the EF-Hand loop sequences stripped from the structural corset. Inorg. Chem. Front., 2022, 9, 4009-4021.
The authors consider that these organisms have the potential to solubilize the transplutonium actinides, in practice Am3+ and Cm3+ after breakdown of containment drums in deep disposal sites. (The solution to this "problem" is to not build deep disposal sites in the first place, but rather to put all of the components of used nuclear fuel to important uses.)
It is worth noting that there are a class of drugs known as ADC's, antibody drug conjugates, that are designed to carry highly radioactive atoms selectively to cancer cells. In this case, radiopharmaceuticals, a very special kind of ADC is prepared. Traditional ADCs carry a chemical payloads to the surface displays of cancer cells, where as their radioactive analogues carry radioactive atoms to the cancer cell surfaces, with a linker attached to a DOTA complex of a radioactive atom, often an isotope of actinium. The point is that if enough Am3+ and Cm3+ is carried in significant amounts, amounts high enough to matter, like cancer cells, the carrying cells are likely to be killed by the payload.
Again though, the point is not to bury actinides, but to praise them, treasure them, and put them to use.
An interesting application for this work would be to engineer immobilized organisms, perhaps mat forming bacteria to display lanmodulin on the the cell surface, and thus produce an effective filter or recovery system for valuable Am3+ and Cm3+.
Have a nice day tomorrow.
Ukraine and Westinghouse sign agreement to Build A Nuclear Plant.
Ukraine and Westinghouse sign agreement for Khmelnitsky AP1000Subtitle:
Excerpts of the article:
Kotin said: "We have reached a very important stage in the process, which was started back in 2021 during the visit of the President of Ukraine, Volodymyr Zelensky, to the USA. I believe that the signing of the contract for the purchase of equipment for the AP1000 power unit is an epoch-making event in the development of the domestic nuclear power industry. The first western power unit in Ukraine will add more than 1100 MW of capacity and strengthen domestic energy independence."
Haluschchenko said the agreement was part of the country moving away from Russian technology in its nuclear energy industry, noting of the AP1000 "such reactors did not exist in Ukraine, they do not exist in the post-Soviet space, and they do not yet exist in Europe ... this is important for strengthening the energy security of Ukraine and renewing our nuclear power industry, which was, is and will be the key generation in Ukraine".
Fragman said: "We are moving to a new level of cooperation. This agreement is of fundamental importance for the energy security of Ukraine, because it is a very reliable technology. Today, five AP1000 units are in operation in the world, in particular in the USA and China, five more are almost ready for start-up. Poland and Bulgaria are interested in the construction of such reactors, as well as a number of other countries."
The go-ahead for the pilot AP1000 project in the country at Khelmnitsky was signed in November 2021, with another one proposed for the same site among the nine new AP1000s planned in total in Ukraine...
Ukraine is planning for a time past the war, and it's nice to see this effort to eliminate dependence on fossil fuels, the chief export of Russia. It is moreover, a serious effort to limit, for the benefit of humanity beyond Ukraine's borders, to address climate change.
Somehow I got on Tammy Murphy's mailing list.
I'm getting a lot of texts and emails.
I'm an Andy Kim kind of guy though.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509