NNadir
NNadir's JournalCan Bisphenol A Be Replaced By a Derivative of Vanilla?
Recently I remarked in this space that one possible route, maybe the only route, to fixing carbon dioxide from the air - something that will prove necessary for future generations since our generation did nothing whatsoever to address climate change - will involve making products from biomass that effective sequester carbon.
The difficulty of taking a lab route for usefully fixing CO2 to an industrial level.
In recent years there have been many discussions of this approach in the scientific literature, and I came across an interesting paper that puts a fun - and interesting - spin on the topic.
The paper is here: Synthesis and Characterization of Bio-based Epoxy Resins Derived from Vanillyl Alcohol (Joseph F. Stanzione III, ACS Sustainable Chem. Eng., 2016, 4 (8), pp 4328–4339)
Many people are aware that there is growing concern that many plastics, in particular polycarbonates, are co-polymerized with bis-phenol A, which can leach out of the plastics and is a known endocrine disrupting chemical owing to some structural features making it similar to some steroidal compounds in the estrogenic pathway. Polycarbonate plastics are the tough, hard plastics commonly used for water storage bottles, baby bottles, etc.
An excerpt from the text of the paper is here:
DGEBA is a product of two main reactants, BPA and epichlorohydrin, with epichlorohydrin, historically synthesized via a multistep pathway starting with propylene.(1, 2, 6, 7) There is currently no renewable source for BPA; however, Dow Glycerin to Epichlorohydrin (GTE) Technologies and Solvay Epicerol have recently reported processes for the synthesis of epichlorohydrin from bio-based glycerol, a byproduct of biodiesel production.(8, 9) BPA (4,4?-isopropylidenediphenol) is typically synthesized via an acid catalyzed electrophilic aromatic condensation of phenol and acetone with a stoichiometric ratio of 2:1, yet the process uses large excesses of phenol to reduce the formation of higher molecular weight oligomers.(10, 11) BPA is used as the base molecule in thermosetting epoxy resins, as the bisphenolic structure provides molecular rigidity to the polymer network; thus, promoting their outstanding thermomechanical properties.(11) However, the use of BPA in epoxy resins has received a great deal of scrutiny and debate, while concerns of human exposure to BPA, a known human endocrine disruptor, via leaching from resins and food and beverage can coatings are driving the search for a suitable alternative that is both renewable and nontoxic.(3, 12)
The Dow process for producing epichlorohydrin from glycerol, a generally worthless side product of the production of biodiesel and soap, is described here:
Glycerin as a Renewable Feedstock for Epichlorohydrin Production. The GTE Process (Briggs et al Clean 2008, 36 (8), 657 – 661)
The idea is to take a "generally worthless" product and make it worth something. Polymers derived from glycerol are fixed carbon that is removed from the atmosphere.
Now for the fun part, the chemical whose flavor I love, vanillin, actually the alcohol made by reducing vanillin.
The authors write:
To produce a bio-based epoxy, BG (3) was then reacted with epichlorohydrin (4) to produce a diglycidyl ether of bisguaiacol (DGEBG; 5) as shown in Scheme 3. To study the influence of the methoxy moiety attached to the aromatic ring on cured polymer properties, diglycidyl ether of vanillyl alcohol (DGEVA) and diglycidyl ether of gastrodigenin (DGEGD) were also synthesized in the same manner as DGEBG (Figure 1). Furthermore, as DGEVA and DGEGD can be considered useful bio-based epoxies, diglycidyl ether of hydroquinone (DGEHQ; Figure 1) was also synthesized in order to study the influence of the methylene spacer between the aromatic ring and the glycidyl ether. The epoxy resins were cured, either by themselves or with a commercial BPA-based epoxy resin, with stoichiometric equivalents of Amicure PACM (4,4?-methylenebiscyclohexanamine; Figure 1). The thermomechanical properties of the cured polymers were tested via dynamic mechanical analysis (DMA) to determine if these resins are suitable alternatives to current commercially available petroleum-based resins.
For those who are interested in organic chemistry, here is scheme 2:

Here is scheme 3, regrettably not high quality as a graphic, but if you know some organic chemistry, you can fill in the blanks:

Recall that the authors are proposing to use epichlorohydrin derived from glycerol, so the molecules in scheme 3 are entirely derived from biomass.
Vanillin is of course available from vanilla beans, but it is mostly available on an industrial scale from the hydrolysis or thermolysis of lignin, the structural component of wood and straw that is not cellulose. (Many similar compounds are also obtained from the digestion of lignin, many of which will prove to have other uses.)
The authors report that the resulting polymers have excellent properties, comparable to the properties of the petroleum based thermosetting polymers.
There's a long way between bench top chemistry and commercial applications, but, nonetheless, it's interesting I think.
I wonder if the leachates will taste good. I personally love the vanilla flavor; can't get enough of it. I'm a vanilla kind of guy.
Have a nice evening.
A Brief Note of Warning on Fatalities Associated With the Storage of Wood Stove Pellets.
This one came as a surprise to me, but I stumbled across it while going through the scientific literature this afternoon. Apparently in recent years there have been nine fatalities associated with the storage of wood pellets for wood pellet stoves.
Influence of Oxygen Availability on off-Gassing Rates of Emissions from Stored Wood Pellets (Irene Sedlmayer et al, Energy Fuels, 2016, 30 (2), pp 1006–1012)
From the text:
Wood pellets are known to emit various gaseous emissions during production, transportation, and storage.(4-9) Nine fatal accidents occurred since 2002 during storage or transportation of mostly big bulks of wood pellets but also in small scale stores.(10) Subsequent investigation indicated increased concentrations of CO, CO2, VOC, CH4,(4, 5, 11, 12) and H2(13) and simultaneous depletion of O2(14, 15) in pellet stores leading to a toxic breathing atmosphere. According to Svedberg et al.(12) O2 declined to levels from 16.9 to 0.8% in closed storages like ocean vessels. However, the concentration of oxygen in ventilated pellet storages strongly depends on the efficiency of ventilation. It can be assumed that in an appropriately ventilated pellet storage room, the oxygen concentration is approximately equal to the oxygen concentration of ambient air.
Reference 10 is here: Lethal Carbon Monoxide Poisoning in Woo dPellet Storerooms—Two Cases and a Review of the Literature (Saskia Gauthier et al, Ann. Occup. Hyg., Vol. 56, No. 7, pp. 755–763, 567755–763 (2012)).
A description of the fatal accidents are found in table 2 of the text. Of the nine deaths, only one was associated with storage in a private home.
My recommendation is that if you're using wood pellet stoves one should store them in a well ventilated space such as a shed or garage that is aerated. They should probably be cycled with the oldest being burned first. A carbon monoxide detector in the area or storage is advisable, particularly if the storage place is confined. It might be wise to store them in a sealed container.
I'm not sure why this is not a problem with the storage of wood for fireplaces. Probably it is related to surface area, since the wood pellets I've seen are small, um, well, pellets.
I have wood burning fireplace in my home with a circulating air fan to heat my home. In recent years I've been less and less inspired to use it - even though I use downed wood from trees on my property that would otherwise rot releasing carbon dioxide - since I am aware that about half of the seven million air pollution deaths that take place each year are from the combustion of biomass, particularly indoors. My home is regrettably heated by dangerous natural gas which is less of an air pollution source than wood, at least in a purely chemotoxic sense, but noxious all the same in a climate sense. About 50% of the electricity supplies in my state is for the time being supplied by nuclear energy, so when possible I do use electric space heaters.
(I'm not sure we're going to have all that many winters in New Jersey in the future, since we bet the planetary atmosphere on so called "renewable energy" with the result that the rate of climate change driving carbon dioxide releases is rising, not falling. So called "renewable energy" didn't work, isn't working and won't work.)
But in any case, if you have a wood pellet stove, be safe.
Enjoy the rest of your Sunday afternoon.
Following cadmium flows in grains and other plants by isotopic fractionation.
Cadmium is highly toxic element that occurs naturally in some soils, but has been increasingly contaminating agricultural fields since the element has been mined extensively in order to serve the electronics industry.
It is known that the element is taken up by plants since it is mimetic for the elemental essential nutriet zinc, zinc being present in the active sites of many important metalloenzymes essential to life. When coordinated with cadmium rather than zinc, the enzymes no longer function, and this is important to the mechanism by which their toxicity is observed. (The third cogener of zinc is mercury, and the mechanism of its toxicity is generally the same: Both cadmium and mercury are powerful neurotoxins, which may account for the unfathomable popularity of Donald Trump.)
An interesting paper I came across on the subject in the current (as of this writing, 9/10/16) issue of Environmental Science and Technology is here: Cadmium Isotope Fractionation in Soil–Wheat Systems (Matthais Wiggenhauser et al, Environ. Sci. Technol., 2016, 50 (17), pp 9223–9231)
Here is a graphic from the paper:

It is important to note that the percentages here do not refer to enrichment of total cadmium in the various plant parts, but rather in the ratio between two stable isotopes of the element, 114Cd and 110Cd, with the former being enriched.
Isotope effects such are these are related to the kinetics of reactions, which vary (slightly) with the mass of the reactants. These effects are well known for the hydrogen isotopes protium, deuterium and tritium, but, as the paper points out in the text, have only in recent times been accessible to study owing to developments in inductively coupled plasma mass spectrometry (ICP-MS). For the analytical chemist, details may be found in the supplementary information of the paper although the particular make and model of the ICP/MS is not noted.
The lighter the isotope, in general, the faster the reaction. In the present case, it was found that the cadmium that makes its way to the wheat seeds, the grain we eat, is enriched in heavier isotopes meaning that the reactions in the stems and roots remove some cadmium before it gets to our mouths and, ultimately our brains where it causes diseases like Trumpism and Greenpeacism and other neurological deficiencies. This is actually good news, since it means that some of the cadmium is sequestered in these inedible parts of grain, although in the case of straw, it may show up in animals that some people eat, in particular, cows.
Despite this marginally good news, it is clear that many food supplies are in fact contaminated with cadmium, especially in China, where electronic recycling, as well as mining and manufacture of semiconductors solar cell are practiced under largely uncontrolled conditions. It is reported that in Southern China, up to 70% of the grains purchased in local markets had cadmium levels exceeding government set limits: F ood supply and food safety issues in China (Sun et al Lancet 2013; 381: 2044–53) About 1/6 of the world supply of cadmium is mined in China; substantial quantities are mined in the United States and isolated from various zinc ores.
We hear a lot of hand waving about how solar prices are dropping dramatically, and Chinese manufacture of solar cells is a big part of the reason, because in China, despite having declared itself a "people's state" environmental regulations either do not exist or if they do, are poorly enforced. In China it is cheaper to dump cadmium containing wastes and avoid the purchase of expensive devices to prevent the escape of these contaminants, as well as other contaminants like tetrachlorosilicon, and yes solar prices are dropping. Whoopeee.
(The big lie about "dropping solar prices" is that any system that requires redundancy is not cheaper; it is in fact, more expensive than a system that operates continuously. Also from both an economic and environmental standpoint, the lifetime of a device matters.)
The solar industry has almost no effect whatsoever on the most exigent environmental crisis of our times, climate change. After the world's population dropped a trillion bucks on this adventure in wishful thinking and poor critical thinking ability, the result is that the rate of accumulation of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere has pretty much tripled since the big mouthed advocates of this scheme - I'm talking specifically about corporate window dresser Amory Lovins specifically, although one could point to similar assholes like his one time acolyte Joe Romm - began telling us all that solar and wind would save us.
They haven't, they aren't, and they won't.
The import of cadmium laced solar cells into the United States from China will probably have little immediate toxicological effect - although I'm not sure the issue has been studied - but in twenty or thirty years all the solar cells on this planet, as well as the inverters and other electronic junk associated with them, will become another component of electronic waste, already an intractable problem. The toxicological effects thus will fall on future generations. By doing nothing but failing to check our 1970's assumptions, the members of this generation, my generation, have showed their contempt for all future generations in thousands of ways, and piling up this solar junk is only the tip of the overheated iceberg.
By the way, cadmium is one of the "critical elements" that are likely to be depleted in the lifetime of today's infants. Here is a periodic table from one source on this increasingly discussed issue in Engineering, Materials Environmental Sciences: The importance of elemental sustainability and critical element recovery (Hunt et al, Green Chem., 2015,17, 1949-1950
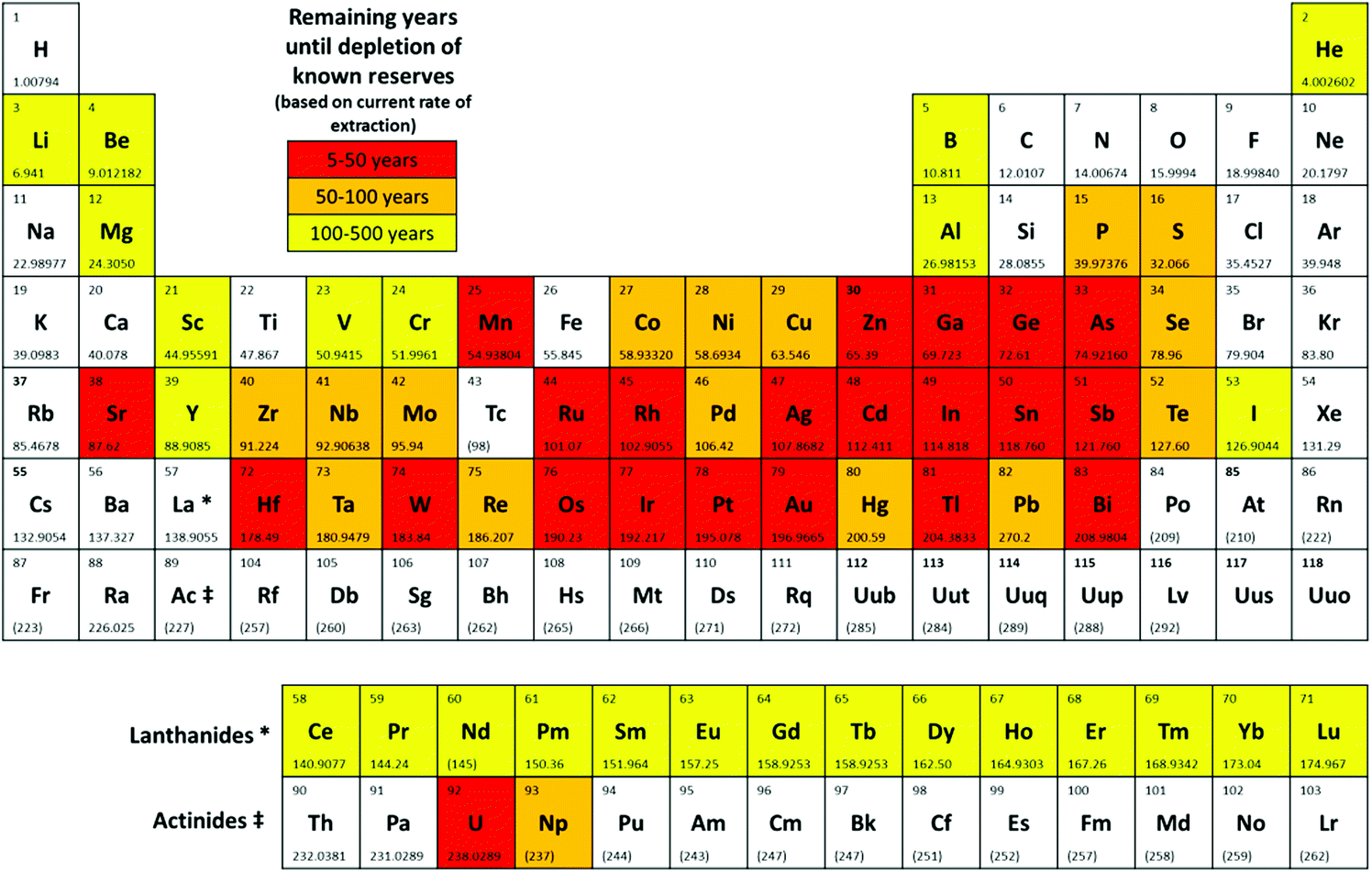
It is somewhat amusing to note that five of the elements that have been touted as "solar breakthroughs" in the last twenty or thirty years of useless cheering, five of them are on the list of immediately endangered elements, those expected to run out in the next fifty years, the aforementioned cadmium, as well as gallium, germanium, indium and arsenic. (Indium may well run out first, not because of useless solar cells, but because of touch screen phones and other screens.) Two others, selenium and tellurium, both toxic elements, will run out in the next century.
So much for the hope that the solar industry, where "PRICES ARE DROPPING!!!!!!!!!" will end up producing all that much more than the less than the 2 exajoules it produces right now out of the 570 exajoules of energy that humanity now consumes each year.
Enjoy the rest of the weekend.
Busted my 128 gig thumb drive yesterday.
It was almost in two pieces - the lap top fell on it when I accidentally knocked it over but with patience and coaxing I managed to get the last week of work off of it since the last back up.
It's amazing what one can get on one of those little things; years of work; and it's so damned fragile.
Over the years, I've left a few in libraries, and thus fortunately, got in the habit of regular back ups.
Sad though, and a little frightening.
A Child Should Be A Fish.
<iframe width="854" height="480" src="
" frameborder="0" allowfullscreen></iframe>Um, how much hydrogen could the $2.2 billion Ivanpah solar thermal plant produce?
This weekend, while catching up on my reading, I came across a carbon dioxide splitting/hydrogen thermochemical cycle of interest.
I have a long term interest in thermochemical hydrogen cycles, and have probably, over the years, read a few hundred papers about them.
The particular paper I was reading was this one: Applicability of an Equilibrium Model To Predict the Conversion of CO2 to CO via the Reduction and Oxidation of a Fixed Bed of Cerium Dioxide (Luke J. Venstrom et al Energy and Fuels, 2015, 29 (12), pp 8168–8177)
The chemistry of this system works like this: Small cylindrical porous particles of cerium dioxide (aka cerium (IV) oxide, CeO2, roughly 3-5 mm in length and 5 mm in diameter, are heated to 1200oC, whereupon a fraction of the CeO2 is reduced to Cerium(III) oxide, Ce2O3, dicerium trioxide. In this process oxygen gas is released.
After the oxygen is removed - and this paper is on the subject of how one might do that - one of two things can be done.
The first is that carbon dioxide, the dangerous fossil fuel waste that is now - as of 2016 - accumulating in the planetary atmosphere at a truly astounding rate - can be passed over the Ce2O3 at which time the carbon dioxide is reduced to carbon monoxide whereupon the Ce2O3 is reoxidized to CeO2. The CeO2 in this case is thus a catalyst; it is returned to its original state. Thus the net reaction is this:
The second is that water in the gas phase, steam, can be passed over the Ce2O3 where upon it is reduced to hydrogen gas, whereupon, again, the Ce2O3 is reoxidized to CeO2, and again he CeO2 in this case is thus a catalyst.
The net reaction in this case is:
Note that one of the world's most practiced industrial reactions - the reaction by which the bulk of the world's hydrogen is currently produced is the water gas reaction:
In this sense, the two paths each represent a path to hydrogen gas, which is useless as a consumer fuel, despite much bull to the contrary thrown around insipidly for the last three or four decades, but is very useful as a captive intermediate for the production of ammonia, and, in some places, liquid fuels ranging from gasoline to diesel to dimethyl ether and other related chemical products normally produced from petroleum.
Mixtures of carbon monoxide and hydrogen have a special name, "syn gas." Using "syn gas" in the golden age of chemistry in which we live, we can make pretty much any industrial scale organic chemical we want.
Now for the fun part. The authors of the paper cited in the opening text write the following:
I mean no criticism of the authors of this fine paper to state that I expect - I hope - they are being disingenuous when they write this line of bull. Science is poorly funded these days, and let's face it, in this cockamamie world if one wants to get a grant, one is better positioned if one puts the word "solar" in the grant proposal.
These papers about solar hydrogen have been flying around for decades and the number of concentrated solar plants on this planet producing industrially meaningful quantities of hydrogen is zero.
Here, for instance, is a link to a paper I randomly pulled up from my files that was published 51 years ago: Solar Energy Volume 9, Issue 1, January–March 1965, Pages 61-67.
Fifty years later, the world's largest solar thermal plant is in California's Mohave desert, the Ivanpah solar thermal plant.
Here are some excerpts from the Wikipedia page about this plant, which pulls few punches - despite the insipid worship of all things solar - on what a grotesque failure this huge piece of garbage has been:
"A gross capacity of 392 megawatts..."
Below we'll take a look at the actual power this plant produces, and compare how many Ivanpah solar plants would be required to match the power output of a 1000 MWe nuclear plant.
First let's look at the cost of the plant:
And the land use:
The plant has never produced enough electricity to meet its contractual delivery requirements which afford it the right to sell electricity to PSEG and SCE for $200/MWh, $50/MWh higher than the retail delivered residential cost of electricity in California.
The plant, by the way, is required to burn dangerous natural gas every morning to start up. The waste from the dangerous natural gas is dumped indiscriminately into the world's favorite waste dump, the planetary atmosphere. The plant has burned approximately 1.8 billion cubic feet of natural gas in its three years of operation.
The power output for the entire facility can be calculated from the data in the table at the bottom of the Wikipedia page, which includes data up to June of this year - the mirrors at the plant went out of alignment in July causing one of the three towers in the plant to catch fire, whereupon the two billion dollar piece of crap was shut for a few weeks.
I have taken the liberty of converting these totals, given in MWh of electricity (solar) into units of average continuous power for the periods listed in the table, in order to give a sense of scale.
In the first month of operation, January 2014, the plant produced an average continuous power of 14 MW, and it did not approach 100 MW until the month of June 2014, when it produced 89 MW of average continuous power. For the entire year of 2014, it was the equivalent of a 47 MW power plant.
Since coming on line, the plant has produced more than 100 MW of average continuous power in only three months: In April of 2015, it produced 104.59 MW of average continuous power; in June of 2015 it produced 107.68 MW of average continuous power; and in February of this year it produced 100.08 MW of average continuous power.
Overall, during it's entire history it has been the equivalent of a 61.11 MW power plant.
Thus, at 2.2 billion dollars in cost, with 1.6 billion dollars represented by loan guarantees by the US government, in order to produce as much power as a 1000 MWe nuclear plant, we would need 16.4 of these disasters, and the cost would be $36 billion. The land area required would be 265 square miles of desert.
The big difference between a nuclear plant and this piece of expensive and useless crap is that the nuclear plant would 1) actually work, 2) would operate for about 60 - 80 years and 3) would not require burning huge amounts of dangerous natural gas to start up, and 4) would not require redundant plants, fueled by dangerous fossil fuels to support it whenever the sun went down. The nuclear plant could produce all of the power of the 265 square miles of solar plant in a moderate sized industrial building.
It is interesting to note, that the cost of educating an (out of state) nuclear engineer at the University of California at Berkeley is roughly a quarter of a million dollars - a fact that sticks in my mind as my two sons are of college age, one in college, and one about to enter college. The $1.6 billion loan guarantee, which may need to be paid since the plant is technically in default on its contract since it has never met its contractual obligations to deliver electricity, is enough to pay for the full educations of 7,500 engineers, not that we give a shit about paying for engineering educations in this country.
By the way, the cerium spitting cycle would be better served by using nuclear heat, which is certainly accessible given recent advances in materials science and which is more reliable.
It is interesting to note that 144Ce is a fission product - unimaginative people with very small minds call this isotope "nuclear waste - with a 284 day half life, decaying through 144Pr to give stable 144Nd. Properly isolated, it could put out significant heat.
It is certainly conceivable to isolate Ce isotopes from continuously fueled fluid phase reactors, not just the famous molten salt reactors that many people are hyping, but from some of the aqueous solution phase reactors of the type originally built and designed by Enrico Fermi. As I recently learned, somewhat to my surprise, 17 examples of these reactors operated at Los Alamos for a period of roughly 20 years from the 1950's through the 1960's until the early 1970s. None of them required 4000 acres of land; all of them in fact, operated in small rooms. They were cheap to build, easy to operate, and apparently very reliable. It is said that Fermi would take breaks from his theoretical studies during the Manhattan project years to go play with one that he built every afternoon. He liked to operate it himself; it is said he'd never let the technicians operate it, since he was fascinated with it, and wanted to be absolutely certain of all of its operating features and thus insisted that he run the thing himself to be aware of everything the reactor did. (It was used to develop an understanding of some of the basic physics of fission, including cross sections of important nuclei.)
So how much hydrogen could the $2.2 billion dollar solar thermal plant at Ivanpah produce? Not enough to count, that's for sure. We sank a trillion dollars into the solar energy industry in the last ten years with the result that we have now tripled the rate at which new carbon dioxide is added to the atmosphere as compared to the rate in the 1970's. The solar industry - at least if you believe that the ends justify the means as opposed to believe that the ends are irrelevant and only the means count - is a grotesque and expensive failure.
Enjoy the labor day holiday.
Photographs of some areas affected by the release of silicon tetrachloride at Chinese plants.

Here's the caption:
(a) SiCl4 incident at Luoyang City, Henan Province. Dump of SiCl4 (left) and wilting corns (right). It occurred on March 9, 2008 and caused the land unavailable to grow crops
and not suitable for people to live nearby [12].
(b) SiCl4 incident at Puyang City, Henan Province. Release of SiCl4 from worn-out tank (left) and emergency treatment of silicon tetrachloride by firemen (right). It occurred
in October 13, 2009 leading the crops in the fields over 30,000 square meters around the release site to suffocated to death by the fumes of SiCl4 [13].
(c) SiCl4 incident at County Xingtang, Hebei Province. Release of SiCl4 from worn-out tank (left) and seared wheat fields (right). In May 19, 2009, a severe silicon tetrachloride
release occurred. The wheat seared in the fields about 80,000 square meters around the release site and tens of residents reported eye and respiratory system deceases [14].
The source is here: Numerical investigation on three-dimensional dispersion and conversion behaviors of silicon tetrachloride release in the atmosphere (Journal of Hazardous Materials Volume 288, 15 May 2015, Pages 1–16)
Have a nice holiday weekend.
The difficulty of taking a lab route for usefully fixing CO2 to an industrial level.
Since the early 1990's, it has been known on a laboratory scale, that one can electrolytichemically reduce carbon dioxide to chemicals usually obtained from dangerous fossil fuels, such as methane.
What is more interesting is the possibility of reducing carbon dioxide to compounds that can be polymerized, such as "ethylene" (formally ethene) which can be polymerized to give plastics like, um, obviously, polyethylene. In this case, since polyethylene is a useful material utilized in many products, this would represent a means of theoretically removing carbon dioxide from the air and sequestering it without relying on an expensive (and thus far, despite all the jawboning about it) unrealized series of massive carbon dioxide dumps to contain the more than 30 billion tons we dump each year, a number which is growing, not falling.
The following graphic is reproduced from a fanciful paper published in 2010, J. Phys. Chem. Lett., 2010, 1 (24), pp 3451–3458 about a magical "scenario" wherein we would produce plenty of electricity and greatly reduce our carbon dioxide output.

The title of the paper published in 2010 was "Prospects of CO2 Utilization via Direct Heterogeneous Electrochemical Reduction"
It's been six years since this rosy "scenario" was put forth, and it is germane to the question to ask whether we are moving in the direction, this "Prospects" paper puts forth.
Um, no we aren't. The fastest growing segment of energy production in the United States, and certainly the world is a dangerous fossil fuel - a very dangerous fossil fuel - dangerous natural gas. Back in July, in a post entitled "The fastest growing source of US electricity has lead to large CO2 reductions for US electricity" I noted this, writing, while referring to one mechanism for producing so called "renewable energy:"
The wind industry, since the source of its power the wind is variable, has not, cannot, and will not exist without backup power, a redundancy which advocates never include in the environmental and financial cost of this essentially useless, but expensive, industry.
Many people want to believe that so called "renewable energy" is working. It isn't. It hasn't. It won't.
Note that the graphic produced above is not, as many advocates of the failed and useless so called "renewable energy" are, hostile to nuclear energy.
In this country, if not in Asia, stupid people have grabbed the energy microphone, pushed forth their deadly and dangerous ideas, and nuclear energy is in decline, not in a growth mode, as the wishful graphic of 2010 hoped it would be.
And frankly, we're out of time. Time's up.
The average comparisons of carbon dioxide increases in comparisons of weeks of 2015 (the worst year ever observed in increases in carbon dioxide) to the same weeks of 2016 is now 3.55 ppm higher than last year. (In 2015, this figure as a comparison to 2014's weeks, was 2.23 ppm, then the highest ever observed.)
The rate at which new carbon dioxide is being added to the atmosphere is dramatically worse than anything we've ever seen before.
Still, I sit at computers reading all about what could have been but wasn't, even though it disgusts me at the deepest ethical level that I've been hearing that word could being abused unmercifully as the disaster goes completely out of control. "We could run the whole world on renewable energy by such and such a year" - the year always being at a time that the issuer of this wishful thinking will be dead.
I suppose that the industrial electrolytic of carbon dioxide to ethylene might work, but it would be a very, very, very stupid - and quite possibly dangerous - idea to look at the idea and be comforted while making wild dogmatic statements about energy.
This is what we have done though. And we failed. And those we have failed are all future generations.
So if we've known for sometime that we can reduce carbon dioxide to ethylene, why haven't we done it?
Well, lots of labs are working on it, as I learned after stumbling upon a paper on the subject that caught my eye, this one: Stable and selective electrochemical reduction of carbon dioxide to ethylene on copper mesocrystals (Catal. Sci. Technol., 2015,5, 161-168)
Here's the problem, according to the paper:
The paper has already been cited 25 times. There seems to be a lot of work on the copper catalyst involving its nanostructure that makes a difference.
Will it go industrial? Well maybe; there's lots of very smart people working on the project.
Will it make a difference? Will it be on time?
No it won't. It's already too late. It's been too late for a fairly long time now, and to the extent we get all excited when we read this stuff, we're lying to ourselves.
Best wishes on the upcoming Labor Day weekend. I hope you will realize any wonderful plans you may have made.
Why the honey badger doesn't care about cobra venom.
Venomous snakes kill up to 94,000 people every year, on top of the millions of other animals that make up their diet. And death by venomous snakebite isn’t pretty: The toxins in venom can paralyze muscles, break down tissue, and even make victims bleed uncontrollably.
So why don’t honey badgers care about venoms that can kill almost any other animal?
Danielle Drabeck, a University of Minnesota grad student, wanted to study this question on a molecular level, but she ran into a problem: Honey badgers aren't found in Minnesota or even the Western Hemisphere, but only in Africa, the Middle East, and India.
Biology Finally Explains Why Honey Badger Don’t Care
My boys love that "Honey Badger Doesn't Give A Shit" video, and I came across it, and wondered how they got away with eating cobras. It appears that they, mongooses, and surprisingly pigs, all have mutations in the protein binding site in the cobra neurotoxin receptor.
<iframe width="640" height="360" src="" frameborder="0" allowfullscreen></iframe>
Superalloy Failure Apparently Had "Painful" Results in the Vietnam War.
I've been leafing through an interesting monograph on Materials Science, specifically "superalloys" with which I've been hanging out for the last few years. Here is a link to the monograph:
Superalloys Alloying and Performance
"Superalloys" are alloys, generally nickel based, that show high mechanical strength at high temperature while being resistant to corrosion (oxidation). They hold an extremely important role in modern technology for use in things like jet engines and power plants operating at high efficiency.
I wrote a riff citing this book elsewhere:
Technetium: Dangerous Nuclear Energy Waste or Essential Strategic Resource?
Anyway, I came across this interesting bit in the text about the failure of superalloys during the Vietnam war, which apparently had some unfortunate consequences:
Um, the Vietnam war itself was "clearly unacceptable," but no matter...
This tidbit is found on page 5 of the text.
That's an interesting bit of history about which I never heard, and I thought other people might find this obscure fact interesting, so I'm posting it.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512