NNadir
NNadir's JournalChemical Principles of Topological Semimetals
In the midst of the White House generated horror of the last days, I had the guilty pleasure of attending my favorite kind of lecture: A lecture that was not only on a subject about which I know nothing, but on a subject about which I never even heard, topological semimetals.
One of my goals in life is to feel as often as is possible like I'm the dumbest person in the room, and I definitely succeeded in this case.
The lecture was given by Dr. Leslie M. Schoop, the newest faculty member of the Princeton University Department of Chemistry.

I immediately went home after the lecture and began to look into the topic and was pleased to see that I recently downloaded (but clearly didn't read) a review article written by Dr. Schoop and her colleagues.
The article, from which the total of this post is taken is here: Chemical Principles of Topological Semimetals (Leslie M. Schoop,*,† Florian Pielnhofer,‡ and Bettina V. Lotsch, Chem. Mater., 2018, 30 (10), pp 3155–3176)
It's a relatively new, if rapidly expanding field, so I guess I can be excused for knowing nothing at all about it, but it apparently involves some novel particle physics apparently predicted by the mathematical physicist Hermann Weyl during the scientifically transcendent 20th century.
Since it involves the structure of matter, I plan to share this with my son when he returns from Europe, I believe he'll find it cool.
Much of the topic remains over my head, but I thought it might be interesting to post brief excerpts of the paper along with some of the beautiful graphics from it.
The practical application, should it ever develop, would be computers so fast as to revolutionize computation as much as the original digital computer did in the 20th century, the elusive quantum computer: At least this is what Dr. Shoop claimed.

The caption:
The intro continues:
...
The review then discusses the remarkable properties of graphene which Dr. Schoop remarked with some amusement can be made by peeling a single layer of carbon atoms off of graphite with masking tape.

The caption:
Some remarks on graphene as a prototype of the "Dirac Semimetal"
Dirac Semimetals
The prototype of a DSM is graphene. The “perfect” DSM has the same electronic structure of graphene; i.e., it should consist of two sets of linearly dispersed bands that cross each other at a single point. Ideally, no other states should interfere at the Fermi level. Note that in a DSM, the bands that cross are spin degenerate, meaning that we would call them two-fold degenerate, and thus the Dirac point is four-fold degenerate. When discussing degeneracies within this Review, we will always refer to spin orbitals. In any crystal that is inversion symmetric and non-magnetic (i.e., time reversal symmetry is present), all bands will always be two-fold degenerate. Time reversal symmetry (T-symmetry) means that a system’s properties do not change if a clock runs backward. A requirement for T-symmetry is that electrons at momentum points k and ?k have opposite spin, which means that the spin has to rotate with k around the Fermi surface since backscattering between k and ?k is forbidden. Introducing a perturbation, e.g., an external magnetic field, lifts the spin degeneracy and violates T-symmetry.

The caption:
"SOC" is spin orbit coupling.
Weyl Semimetals:
The difference between a DSM and a WSM is that, in the latter, the crossing point is only two-fold degenerate.(28,93,94) This is because in WSMs the bands are spin split; thus each band is only singly degenerate. If a spin-up band and a spin-down band cross, this results in a Weyl monopole, meaning that there is a chirality assigned to this crossing. Since there cannot be a net chirality in the crystals, Weyl cones always come in pairs. The resulting Weyl Fermions are chiral in nature and thus will behave physically different from “regular” Fermions. One example of this manifestation is the chiral anomaly, which we will discuss in the Properties and Applications of TSMs section below. Here, we will focus on the requirements necessary to realize a WSM.
In order to have spin split bands, we cannot have inversion (I) and time-reversal (T) symmetry at the same time, since the combination of these two symmetries will always force all bands to be doubly degenerate. In I asymmetric, i.e., non-centrosymmetric crystals, this degeneracy can be lifted with the help of SOC; this is the so-called Dresselhaus effect.(95)

The caption:
Figures for a 3D Dirac Semimetal, trisodium bismuthide, a Zintl salt (at least I knew about Zintl salts for the lecture):

The caption:
A Weyl Semimetal:

The caption:
A "Non-symmorphic Topological Semimetal: "

The caption:
And now, to generate some interest in saving the world after Elon Musk is done saving the world, a possible application, the ever popular solar hydrogen:

Well, at least the degeneracy here doesn't involve that awful excuse for a human being in the White House.
A little interesting if still obscure, at least to me, science is a great way to escape. It's a pleasure to be the dumbest guy in the room, really a pleasure.
I wish you a pleasant day tomorrow.
The greatest car ever, the car that saved all life on earth, spontaneously ignites.
Tesla spontaneously catches fire with no crash
It's green. It's solar. It's wind turbiney. It's the savior of the common man. We need this car more than life itself. The entire US budget should be devoted to its worship.
People Get Ready.
< src="
" frameborder="0" allow="autoplay; encrypted-media" allowfullscreen>2017 Establishes a New Record for Coal and For So Called "Renewables."
The data comes from the BP Statistical Report, which is generally more current than the WEO (published each November) but perhaps not as accurate.
I've downloaded all the data from the BP Report nonetheless, and am going through it. It's an interesting read, showing that things are every bit as bad as I've come to believe, maybe even worse, particularly since energy and environmental issues are filled with so much wishful thinking, outright delusion, and denial on both ends of the political spectrum, this in a world where the center is disappearing.
Other big "winners," besides so called "renewables" and coal were oil and gas, and oh, yes, carbon dioxide emissions.
Carbon Brief: BP Global Data Shows Record Highs for Coal Power
There's a certain amount of "percent talk" here about so called "renewable energy," which is tightly linked to the use of dangerous fossil fuels.
The so called "renewable energy" industry remains what it has always been, trivial, outside of "percent talk" compared to dangerous fossil fuels, and is clearly incompetent to stop their growth.
Oh my God! I was there last evening. This is terrible at a beautiful community event.
My son had a painting on display there.
This is horrible, particularly because "Mothers against gun" had a display there.
Screw the NRA.
Kinetic Modeling of the Sulfur Iodine Process for Thermochemical Water Splitting to Produce Hydrogen
The paper from the primary scientific literature in this post is this one: Building and Verifying a Model for Mass Transfer and Reaction Kinetics of the Bunsen Reaction in the Iodine–Sulfur Process (Zhang et al Ind. Eng. Chem. Res., 2018, 57 (23), pp 7771–7782).
The "Sulfur Iodine Process" sometimes called the "Sulfur Iodine Cycle" or "SI cycle" or (herein) the "IS process" is a process for splitting water using heat, and thus is vastly thermodynamically more efficient than electrolysis and almost infinitely cleaner, depending on the source of heat, the primary energy, than the process by which 99% of the hydrogen on this planet is produced today, the steam reforming of dangerous natural gas or dangerous coal.
There are many thermochemical water splitting processes by the way, and over the years I've familiarized myself with many of them.
The paper is product of scientists at Institute of Nuclear and New Energy Technology, Tsinghua University, Collaborative Innovation Center of Advanced Nuclear Energy Technology, Beijing, 100084, China. For those who don't know, in international rankings, Tsinghua is known to be one of the greatest universities on this planet, and is sometimes ranked in international rankings higher than MIT, depending on the ranking criteria. The people who do research there are smarter than I am, and, I'm sure in many cases among readers, smarter than you are.
Nevertheless, I still feel free to disagree with the last sentence in their opening paragraph:
I personally believe other thermochemical cycles may be more promising, including some involving boiling metals or nanoceramics in flow cells, but that's just my opinion, and again, I'm not that smart.
In any case a 10MW high temperature gas cooled nuclear reactor has operated at Tsinghua University since the year 2000. It's a "pebble bed" type reactor modeled on German technology developed before Germany went "Energy Stupid." It's not my favorite kind of nuclear reactor, but it works.
The Chinese are smarter than we are because they built the reactor in the first place, and it was a new reactor in this century.
I had heard that Chinese scientists were going to fit this reactor to demonstrate the "SI cycle," but haven't kept up with progress in that area, but apparently the process is still getting significant consideration there, as demonstrated in this very recent paper.
The authors describe the "IS process" thusly:
The IS process consists of the following three chemical reactions: (5)
Bunsen reaction: I2 + SO2 + 2H2O = H2SO4 + 2HI
HI decomposition: 2HI = H2 + I2
Sulfuric acid decomposition: H2SO4 = SO2 + 1/2O2 + H2O
The net reaction of the above-mentioned chemical reactions is water decomposition (H2O = H2 + 1/2O2).
Actually in many accounts, what is called the Bunsen reaction above can be actually divided into two separate reactions with the intermediate being sulfuryl iodide, SO2I2, not to be confused with thionyl iodide, a sulfur species in a lower oxidation state. In theory and perhaps in practice, this intermediate could be isolated. I believe that like its chlorine analog, it's a distillable liquid.
An putative advantage of the SI cycle is that most of the materials in it are either liquids or gases, with the possible exception of iodine, although if you have ever worked with free iodine, you have noted that it appreciably sublimes, a gas phase is always above the solid phase, a situation that is also observed with liquid elemental bromine. (There are many variants of bromine based thermochemical cycles by the way.)
The authors discuss these properties, the phase related systems in their text considering how these phase relations affect kinetics that is, the speed at which the process can operate, which is the focus of their beautiful paper. They write, describing the focus of their work:
A graphic from the paper touches on some issues with phase behavior that they examine:

The caption:
In phase interfaces in chemical processes surface area plays a huge role, and hence the discussion of thin films.
The authors construct models for various aspects involved in the kinetics of this system and produce some graphics involved in considerations of various reaction conditions comparing the model with experimental conditions obtained.
For example:

The caption:
...and...

The caption:
Then there are some Henry's law graphics about mass transfer:

The caption:
concentrations.
More mass transfer:

The caption:
Figure 8. Comparison between the model and the experimental results under different initial pressures and I2 concentrations.
Figure
If you are a scientist you already know this, but if you aren't, and find this topic interesting, this might be helpful.
Anything that is flammable is thermodynamically unstable. Wood, for example, in an oxygen atmosphere would rather be carbon dioxide and water, but is stuck being wood because of kinetics, and kinetics in turn is determined by activation energy required to start it. When you burn wood, you provide this activation by using a match, and the match in turn, is ignited by the energy input from friction when you strike it. Because some thermodynamically favorable reactions require energy inputs to get them started, they are able to persist for long periods of time, we say they are "metastable." You by the way, are metastable. So are diamonds at ordinary temperatures and pressures; diamonds are not forever.
One of the first Nobel Laureates, Svante Arrhenius, found a way to determine the activation energy to make thermodynamically favorable reactions like the combustion of wood happen. It is called the Arrhenius equation, after its discoverer and it remains more than a century after its discovery one of the most important equations there is. It is exponential in format, but is often treated as its natural logarithm which makes it essentially linear:
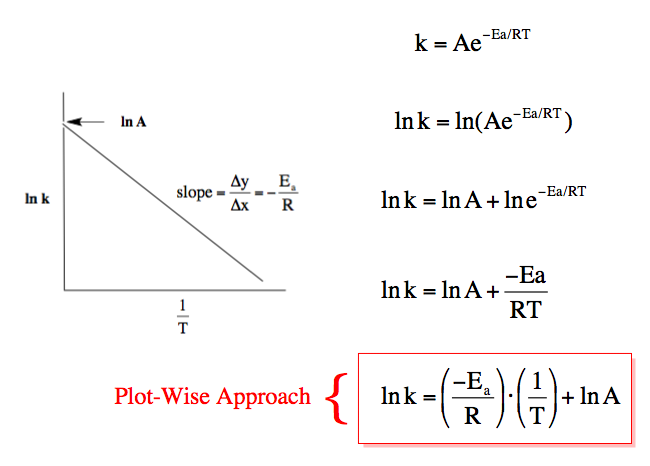
The authors construct Arrhenius plots for the Bunsen reaction:

The caption:
And they find the rate equation for the reaction:

The authors conclude:
Whether you believe it or not - despite whatever horseshit you've heard - the conversion of nuclear energy into chemical energy without the intermediate use of electricity is one of the key technologies for the creation of a sustainable and just world, which is, regrettably, not even close to the world in which we live.
These Chinese scientists irrespective of who pays them or the government under which they live are working in service of humanity, even as the world lives increasingly under a dictatorship of self serving mindless fools.
I am thankful this work is being done.
I am having the best Father's day in my life as a father, since both my sons are doing very well at the things they love, which is all for which a father can hope. If you are a father, I wish you the same.
Large-Scale Uranium Contamination of Groundwater Resources in India.
The paper to which I will refer has the same title as this post, and is published in the current issue of rapid communications environmental journal published by the American Chemical Society. It is this one: Large-Scale Uranium Contamination of Groundwater Resources in India (Vengosh et al Environ. Sci. Technol. Lett., 2018, 5 (6), pp 341–347.
Some text from the introduction to the paper:
Uranium’s threat to human health comes from its chemical rather than it's radiological properties. Epidemiological and toxicological studies have examined the link between the prevalence of uranium in water and chronic kidney disease (CKD) and demonstrated that exposure to uranium through drinking water is associated with nephrotoxic effects.10-12
The authors produce a map as a graphic to show where the problem is worst:

The caption:
30 ?g/L is the cutoff in the World Health Organization's provisional guideline for uranium concentrations in drinking water which is or was the equivalent standard at the EPA, at least before it was taken over a corrupt politician who hates science, scientists and the environment and is treating that organization as a personal bank for corrupt politicians who hate science, scientists and the environment.
Note that the authors attach this situation to geological formations, and that this situation is not involved with uranium mining but with the apparent occurrence of uranium ores through which the drinking water percolates.
This does not, however, imply that human activities have no connection to this situation, which the authors note.
The ocean contains about 5 billion tons of uranium, albeit in considerably lower concentrations than is found in the water supplies studied here. If we take the density of seawater to be 1030 kg/m^3, the figures given in this paper, which I pulled up more or less at random from such papers in my files Chemical Geology Volume 190, Issues 1–4, 30 October 2002, Pages 45-67, we can calculate that the concentration of uranium in seawater is about an order of magnitude lower, 3.4 ?g/L. Many scientific publications give a figure close to this, with minor fluctuations owing to fluctuations in the density of seawater, which is not constant in all places.
Despite all of the talk from people who hate nuclear energy because they know nothing at all about the subject, who have appealed to "peak uranium" to claim that nuclear energy is not sustainable, uranium is not exhaustible, and no human technology can ever consume it.
It is easy to show that if world per capita average power consumption doubled to around 5000W, (which is still half of the average power consumption of an "average" American), that a person living for 100 years would consume about 100 grams of uranium (converted to plutonium) in their entire lifetime. An appreciation of how much uranium has already been mined shows that the quantity is sufficient to supply 100% of humanity's energy consumption for several centuries, even without the greater quantities of thorium that have been mined and dumped as a side product of the lanthanide industry that supports our stupid, environmentally unacceptable and useless wind industry, among other industries.
The recovery of oceanic uranium for use in nuclear reactors has been under study for more than half a century and the technology is well understood. Because uranium is so cheap from terrestrial ores (and would be even cheaper were we to do away with the stupid practice of dumping so called "depleted uranium" ) the cost of recovering uranium from seawater is not justified as of yet, but since in terms of cost per unit of energy provided, the cost of uranium is trivial with respect to the cost of nuclear energy. Like the cost of the useless and failed solar energy industry, the cost of fuel doesn't matter all that much; it's the device that counts.
If at some point stupid people stop ruling the world, and thus the world energy supply goes nuclear, several hundred years from now, people might be inclined to obtain their uranium from seawater, which is possible because of the extremely high energy density of uranium. (Since it is this factor, the energy to mass ratio, is the most important in determining the environmental impact of a form of energy and its economic viability and sustainability, it follows that nuclear fission is the cleanest form of energy possible, unless of course, as had yet to happen, a viable fusion energy device is made to work.) But one might argue that doing this, obtaining uranium from seawater, one could drain the seas of its uranium.
This however is not possible, because the uranium in seawater is actually a tiny fraction of the uranium on the planet as a whole and in fact represents a small part of a natural uranium cycle.
This brings me back to India.
Most people who have studied nuclear issues and nuclear policy - this excludes 99% of nuclear energy opponents, the overwhelming majority of whom argue from ignorance - will understand that the Indian nuclear energy program is geared to utilizing India's large thorium reserves, primarily because India has very few reserves of terrestrial ores of uranium that can be recovered at current low prices. For this reason the majority of nuclear reactors in India are heavy water reactors, which are suitable for breeding U-233 from thorium. However the solubility of thorium is rather low in most aqueous systems (although this is not the case for its radioactive decay daughters). This means that if one considers eternity a thorium/uranium cycle is not sustainable but a uranium/plutonium cycle is.
Since uranium is not present in Indian ores, they have actually built a pilot plant to extract uranium from seawater. Here's a picture of it:

(Source: Linfeng Rao, LBNL Paper LBNL-4034E (2010))
In a blog post elsewhere, I examined the uranium cycle in some detail and using one of the references I supplied therein, among many others, U-Th-Ra Fractionation During Weathering and River Transport (Chabuax et al, Reviews in Mineralogy and Geochemistry (2003) 52 (1): 533-576) A nice table in the paper gives the quantities of uranium transported by rivers to the sea from the weathering of rocks. Three of the top five are major rivers in India: They are the Indus, the Ganges, and the Brahmaputra rivers, with the other two in the top five being the Mississippi and the Yangtze.
Sustaining the Wind, Part 3, Is Uranium Exhaustible?
Now let's return to the paper cited in the beginning of this post.
The authors note that the mobilization of uranium into the ground water is only possible if two conditions are met. One is the presence of carbonate, because in the ocean and in any other aqueous system this solubility is tied to the carbonate complex. The other is the presence of an oxidizing agent, since the carbonate complex of uranium (VI) is soluble, and the same complex of uranium (IV), the other common oxidation state in terrestrial uranium ores is not.
They write:
Additionally, previous studies have observed massive groundwater table declines in many areas in the unconfined alluvial aquifers of northwestern India.5 This hydrogeological condition likely promotes oxic conditions, which favor the occurrence of uranium as a soluble complex and migration into deeper parts of the aquifer. Although neither oxidation?reduction potential nor dissolved oxygen concentration was directly measured, relatively low concentrations of both iron and manganese and high nitrate concentrations further support our hypothesis of oxidizing conditions in the shallow U-rich groundwater (Table S2).
They note that the conditions under which the uranium is mobilized are obtained by the percolation of water through agricultural fields, particularly because of the accumulation of nitrate, as well as the effect of cycling water through the air, which is increasingly concentrated with the dangerous fossil fuel waste carbon dioxide while we all wait, like Godot, for the grand so called "renewable energy" fantasy to become reality.
They have a nice graphic discussing carbonate and uranium fluxes in drinking and agricultural water:

The caption:
That the source of the uranium derives from naturally occurring rocks and not from human industrial nuclear practice is indicated by the U234/U238 ratio since U234, always in equilibrium with U238 is mobilized by the recoil of alpha decay. A graphic on that point:

The caption:
I believe that the value of "1" here refers to the normal secular equilibrium conditions, and not the actual ratio between U234 and U238.
I have written here that the extraction of uranium from seawater is not economically justifiable, and I certainly consider that the nuclear enterprise in a rational world as opposed to the one in we actually live would be powered essentially by so called "depleted uranium" with a little thorium thrown in to keep up supplies of neptunium to void nuclear weapons proliferation issues.
But the question is whether there is a moral and health reason to do it beyond the cost of ores.
Suppose the Indian government decided to purify the groundwater to remove the uranium it extracts from geological formations. Perhaps some of the cost might well be defrayed by selling or utilizing the uranium so recovered. There is no good reason that any of the many systems known to extract uranium from dilute solutions could not be used to remove uranium from drinking and agricultural water instead of seawater. And indeed, the higher concentrations in this water when compared to seawater would make the economics less onerous.
It's worth a shot.
Later, maybe this weekend, I hope to write a post, in response to an excellent question in one of my earlier posts here, to cover the "external costs" of dangerous natural gas, which will show despite common parlance, including much of it by idiotic anti-nukes who claim as evidence of their stupidity that "nuclear energy is not competitive," that natural gas is not cheap since it incurs a health and environmental cost that will fall mostly on future generations, who will have derived none of the benefits of the "cheap" natural gas now being burned in a sybaritic fashion by people with no concern whatsoever about the future.
The authors of the paper from which this post takes its title specifically mention climate change as a factor in the situation with respect to uranium in Indian drinking water. Of course, I assume that every time the words "uranium contamination" appear, the usual anti-nukes perk up their selectively attentive ears to find another insipid "argument" to criticize the nuclear industry. But to whom does the "external cost" of the uranium in Indian water actually accrue? Could it be that some of the moral responsibility lies with those who either deny climate change or propose silly failed schemes to address it?
I pose this question as I finish up by wishing you a very pleasant weekend, a pleasant "Father's Day," if you are involved in some way with a father.
Bats in the Anthropocene.
Many years ago, when my two sons were small, our neighbor used to invite us over to his house on summer evenings for drinks and conversation. Our kids kind of grew up to be different kinds of men, and we got busy and we sort of fell out of touch not because we didn't like each other - we still greet each other very warmly when we do see each other, but because...well, you know, "responsibilities..."
One such summer, a colony of bats moved into the rafters of his house, and at the crepuscule, the bats would come out, swarming and eating mosquitoes. Of course I couldn't tell much about the bats, they were shadows against the colored dying light on the horizon, but I remember how beautiful they were, God they were beautiful.
Some time back, in this space, I referred to a book I had just added to my collection called "Why Birds Matter," which was a book which I claimed justified the existence of birds on economic grounds.
We are so pathetic...
A Minor Problem For Sound Science of the Effect of Offshore Windfarms on Seabirds: There Isn't Any.
The wind industry is a trivial industry that is material intensive, unreliable, ineffective, expensive and dependent on the continuous manufacture of transient junk that last just a short time before becoming landfill, the ultimate consumerist bourgeois exercise in planned obsolescence that is designed to "make jobs" that are not only unproductive, but are actually destructive.
Despite half a century of cheering, and the expenditure of trillion dollar quantities of resources, climate change is worse than ever and we are using more dangerous fossil fuels than we have ever used.
Despite the obvious failure of this awful experiment there are still people who believe that every bit of open space should be turned into industrial parks for producing electricity in a way requiring redundancy and, as I will point out by reference to my latest edition to my collection of books on wildlife, destructive to an important element of the worldwide ecosystem, the creatures I evoked at the beginning, bats.
Before pointing to the book, let me point to a relatively recent paper from the primary scientific literature that states the problem quite clearly and well:
Behavior of bats at wind turbines (Cryan et al PNAS October 21, 2014. 111 (42) 15126-15131). This paper seems to be open sourced, but I'll excerpt it anyway:
...Bats are long-lived mammals with low reproductive potential and require high adult survivorship to maintain populations (1, 2). The recent phenomenon of widespread fatalities of bats at utility scale wind turbines represents a new hazard with the potential to detrimentally affect entire populations (3, 4). Bat fatalities have been found at wind turbines on several continents (3??–6), with hypothesized estimates of fatalities in some regions ranging into the tens to hundreds of thousands of bats per year (4, 6). Before recent observations of dead bats beneath wind turbines, fatal collisions of bats with tall structures had been rarely recorded (7). Most fatalities reported from turbines in the United States, Canada, and Europe are of species that evolved to roost primarily in trees during much of the year (“tree bats”), some of which migrate long distances in spring and late summer to autumn (8). In North America, tree bats compose more than three-quarters of the reported bat fatalities found at wind-energy sites (6, 9), although there is a paucity of information from the southwestern United States and Mexico. Similar patterns occur in Europe (4). Another prominent pattern in bat fatality data from northern temperate zones is that most fatalities are found during late summer and autumn, sometimes with a much smaller peak of fatality in spring (4, 6). Concurrent involvement of species with shared behaviors suggests that behavior plays a key role in the susceptibility of bats to wind turbines, and that tree bats might somehow be attracted to wind turbines (8).
Don't worry, be happy. Wind turbines are "green" even if they have done absolutely nothing at all, zilch, zip, zero to arrest climate change, which is now taking place, after half a century of cheering for wind, at the fastest rate ever observed.
It's not results that count; it's "good" intentions.
The book I just downloaded is this one: Bats in the Anthropocene: Conservation of Bats in a Changing World
Apparently you can download this book for free. God bless Springer publishing, I love them.
An excerpt:
The authors then ask the question, "Why should we care about bats."
There's some nice and gracious lip service to the beauty of bats before we hear about the only thing we care about, money.
Recent attempts to critically review the ecosystem services provided by bats have revealed that many species offer unique and large-scale monetary benefits to agricultural industry (Kunz et al. 2011; Ghanem and Voigt 2012; Maas et al. 2015). For example, flowers of the Durian tree are only effectively pollinated by the Dawn bat, Eonycteris spelaea, in Southeast Asia (Bumrungsri et al. 2009). Durian is a highly valued fruit in Asia with Thailand producing a market value of durians of almost 600 million US$ annually (Ghanem and Voigt 2012). Other bats consume large amounts of pest insects, thereby offering services that could save millions of US$ for national industries (Boyles et al. 2011; Wanger et al. 2014). However, the monetary approach for protecting bat species is a double edged sword, since bat species without apparent use for human economy may not benefit from protection compared to those that provide some ecosystem services.
We are so pathetic...
Chapter 11 of this book is all about the high number of bat deaths at wind turbines, which is entirely OK because we need to grow the wind industry by zillions of percent because, who gives a shit about bats when we can all be "green" and drive swell electric cars made by the ever popular Elon Musk?
In this book, wind power is discussed as one of "the fastest growing sources of energy" even though it, um, isn't, and grew at 1/10th the rate of coal in the 21st century.
IEA 2017 World Energy Outlook, Table 2.2 page 79
But even if its useless, it's pretty good at killing bats.
However returning to accurate statements about their area of expertise, bats, even if they apparently don't know very much about energy they write:
Regardless of causal mechanisms, bat fatalities raise serious concerns about population-level impacts because bats are long-lived and have exceptionally low reproductive rates, and their population growth is relatively slow, which limits their ability to recover from declines and maintain sustainable populations (Barclay and Harder 2003). Additionally, other sources of mortality cumulatively threaten many populations. For example, white-nosed syndrome causes devastating declines in bat populations in the USA and Canada (e.g., Frick et al. 2010), and national programs for improving insulation of buildings, particularly in Northern Europe, cause losses of roosting opportunities for bats such as the common pipistrelle (Pipistrellus pipistrellus; Voigt et al. 2016). Thus, high wind turbine mortality poses a serious threat to bats unless solutions are developed and implemented...
There's plenty more where that comes from.
You know, when I was a kid, I was a member of the Sierra Club, which I then took - it may have been true then - to be an organization dedicated to saving the open spaces, habitat and ecosystems. And of course, I did that thing that clueless bourgeois types like me do, which was to dutifully drive to a shopping mall every Christmas season to get that de rigeur Sierra club calendar with all the rock formations, stream and forest pictures, all printed on recycled paper.
Eventually whether you like it or not, most boys grow up to be men.
That's not what the Sierra Club is today. Recently I attended the New Jersey "March for Science" which turned out to be, to my disgust, a "March for Renewable Energy" where I had to listen to the drivel of the asshole who heads that organization whose "environmental program" calls for destroying the offshore Benthic zone of New Jersey by turning it into an industrial park for wind turbines.
This so called "environmental" organization actually has photographs of a wind industrial park on its web page, at crepuscule no less, the perfect time to kill New Jersey bats.
To the modern New Jersey Sierra club, birds don't matter, nothing matters other than producing electricity some of the time using inefficient material intensive and most importantly useless.
The pathetic asshole who apparently hates bats is in the picture wearing a blue tie. He is, if you must know, one of the most ignorant people you can ever meet, that is if you, unlike him, actually know something about the environment.
Glenn Seaborg, winner of the Nobel Prize, adviser to every President from Harry Truman to Bill Clinton, former chancellor of the University of California, President of the American Chemical Society, discoverer of the final shape of the periodic table, discoverer of more than 10 elements in the periodic table, and Chairman of the Atomic Energy Commission during the most productive period of nuclear reactor building in world history, lead by the United States, was a member of the Sierra Club.
I can't speak for the great man, but I wouldn't be surprised if he would be as disgusted as I am by what that club has become.
I wish you a pleasant Tuesday.
All the World's Coal Plants, Mapped.
All the World's Coal Plants MappedThis is an interactive map, and when it comes up, it will show 2017 on the slider bar.
You may have heard somewhere coal is dead. Maybe you believed it, or at least wanted to believe it.
Expand to Europe, push the slider bar to "Future" from 2017.
Take a look at China and India.
The US, a country of myopic provincials, is closing coal plants because in the US dangerous natural gas is believed to be "cheap." It isn't. It only appears so because the external costs of gas will fall on future generations who will conversely reap none, absolutely none of the benefits.
I wish you a pleasant work week.
Ameliorating the Fire Risk of Energy Storage Devices.
The paper I will discuss in this link from the primary scientific literature is this one:
Promising and Reversible Electrolyte with Thermal Switching Behavior for Safer Electrochemical Storage Devices (Xu et al, ACS Appl. Mater. Interfaces, 2018, 10 (8), pp 7171–7179)
As I frequently point out energy storage wastes energy, a consequence of the inviolable second law of thermodynamics, a law of physical science that all the wishful thinking in the world cannot repeal.
Nevertheless, like so called "renewable energy" itself, the idea of energy storage is inexplicably popular, perhaps because even the most enthusiastic and delusional partisans of so called "renewable energy" recognize, at least in the back of their minds if not consciously, that there are times when the wind does not blow at night. Since they are under the erroneous opinion that so called "renewable energy" is "green" and sustainable, they like to pretend that there is a simple solution in energy storage, and do all kinds of cheering for absurd stuff like Elon Musk's crappy car for billionaires and millionaires.
So called "renewable energy" has not worked; it is not working and it will not work to address climate change or reduce the use of dangerous fossil fuels.
On the other hand, energy storage devices are in fact commercial and work quite well, and are utilized on a grand scale in all sorts of devices, the overwhelming majority of them being portable devices even though, again, they waste energy. The idea of making portable devices however into utility scale devices, while already in some marginal practice to support the marginal and insignificant so called "renewable energy" devices is dubious, especially when one considers that large scale devices, since they waste energy, they can and sometimes do, exhibit heat exchange problems that can become uncontrolled and lead to fires and even explosions.
News item: Lithium-Ion Battery on Delta Air Lines Flight Explodes, Catches Fire
The ignition of batteries brought down UPS Flight 6 in Dubai, killing both pilots, one by asphyxiation, the other being killed when he couldn't land the plane because of smoke. It was covered in the wonderful if scary Smithsonian Channel Show Air Disasters.
News Item: Why the Fire that Incinerated a Tesla Was Such a Nightmare to Put Out
Reducing this risk is the topic of the paper cited at the beginning of this post.
Some commentary from the introduction to the paper:
A series of exothermic reactions leading to a rapid rise in temperature and to thermal runaway can be initiated in addition to the charge/discharge cycle, including the thermal pyrolysis of electrodes and evolution of oxygen and hydrogen between the electrode/electrolyte interface, which in turn increases the internal cell temperature and pressure.11 Accordingly, effective suppression of thermal runaway is the premise of achieving safety application and plays a very important role in the research studies of high-energy storage devices.12
You hear people talking as if battery storage and so called "renewable energy" are already mainstream. They are not. They are trivial and if one believes these scientists - contempt for scientists and engineers is very popular on both political extremes - there are serious technical "breakthroughs" required.
The question that should arise in people's minds is therefore a question of time. It's 2018. We're at close to 412 ppm for the concentration of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere. We cannot continue - if, in spite of all evidence, we want to have any hope of elevating our ghastly impoverished moral standing - to bet the future on hopes for "breakthroughs." We're out of time to wait for them.
Nevertheless, the authors are doing what good scientists do, they are working to solve the problem that the existing problem represents. Their work involves "thermoresponsive polymers." The idea is to shut down the device before it gets too hot and catches fire or explodes. (It's better to have your swell Tesla car stall and be even more useless than it is to have it catch fire and kill people.)
They describe the current state of affairs:
Then they discuss their approach:
Methyl cellulose can be obtained of course from wood and other biological materials and to the extent biological sources are used, this material might represent economically viable sequestration of atmospheric carbon, probably in amounts that would be trivial compared to our 35 billion ton dumping practices in use today, but perhaps meaningful in a world where carbon dumping was arrested by the expanded development and embrace of nuclear energy.
They then produce a graphic showing how their system is designed to work:

The caption:
Here are the cyclic voltammetry (CV) curves showing the reversibility of charge and discharge, a property essential to make a viable battery:

The caption:
Figure 2. Electrochemical properties of AC electrodes in 2 wt % MC-based electrolyte (1 M H2SO4). The CV curves were performed at scan rates of 10, 50, and 100 mV/s on AC electrodes at (a) 25 and (b) 70 °C. The charge/discharge characteristics in reversible electrolyte using a current density of 3 A g?1 at (c) RT, 25 °C and (d) HT, 70 °C.
They test the shutdown of current in terms of heat responsiveness:

The caption:
The DSC curve, curve C, is a differential scanning calorimetry curve, which shows the transition temperature at which the electrolyte converts from a liquid into a gel. This temperature is comfortably low.
And finally, a nice picture of stuff in their lab by which they do the testing:

The caption:
Interesting work, I think. If we must waste energy by storing it, let's try to do it safely at least.
Now of course, I fully recognize that our concept of "safety" is a function of selective attention. I recall a mindless fool here, for example - whose happily made it to my "ignore" list - who focused on the "major news" that a tunnel at the Hanford Nuclear Weapons site collapsed, which in his tiny brain "proved" that nuclear energy was "unsafe," while so called "renewable energy" was without risk.
And of course, people couldn't care less if Tesla cars for billionaires and millionaires catch fire, or if computer batteries bring down planes.
I submit that this kind of thinking is the reason we are at 412 ppm and out of time. It didn't have to be this way, but it is.
All this said, I applaud the fact that these Chinese scientists, if not the general public here in the United States and indeed around the world, are paying attention to risks that are real as opposed to those that are inflated.
Have a pleasant Sunday afternoon.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,513