NNadir
NNadir's JournalAnother Discussion of Biomass Derived Anodes to Replace Petroleum Coke in Aluminum Production.
The paper I will discuss in this post is this one: Renewable Biomass-Derived Coke with Texture Suitable for Aluminum Smelting Anodes (Yaseen Elkasabi*† , Hans Darmstadt‡, and Akwasi A. Boateng, ACS Sustainable Chem. Eng., 2018, 6 (10), pp 13324–13331)
This is a follow up to recent post in this space, Can Biocoke Address the Anode CO2 Problem (Owing to Petroleum Coke) for Aluminum Production?
I don't buy into the pop enthusiasm for so called "renewable energy," since I am aware of the demonstrated fact - the demonstration being the concentration of carbon dioxide in the planetary atmosphere rising at an unprecedented rate despite the "investment" of trillions of dollars on this pixilated adventure - that so called "renewable energy" has not worked, is not working and will not work to address climate change.
Thus, the subtext of my previous post was an inferential attack on this pop enthusiasm, since I noted that the construction of so called "renewable energy" infrastructure is metal (and, just as bad, concrete intensive) and that two of the most important structural metals, specifically steel and aluminum, both depend on access to coke made from dangerous fossil fuels, coal based coke in the case of steel, petroleum based coke in the case of aluminum.
Ironically I am somewhat less hostile to what is clearly the most dangerous form of so called "renewable energy," biofuels than I am to wind and solar, even though biomass combustion is responsible for about half of the seven million air pollution deaths each year. (Recent publications however suggest that while the overall death toll from air pollution is rising significantly, both the fraction caused by biomass and the absolute numbers associated with biomass related deaths are falling, probably owing to improvements in stoves in impoverished areas. Impoverished areas are areas where "renewable energy" never really went away after largely being abandoned by the wealthier population beginning in the early 19th century, when the invention of steam engines made it possible to drain coal mines. Pretty much all of the increasing death toll related to air pollution derives from the rising use of dangerous fossil fuels.)
The reason that I'm less hostile to biomass than I am to wind and solar is that I believe it is technologically feasible to utilize (some) biomass to capture some of the dangerous fossil fuel waste carbon dioxide from our currently unrestrictedly utilized waste dump for it, the planetary atmosphere.
Although abandoning the "renewables will save us" fantasy will prevent massive surges in the demand for steel and aluminum - at what will be in my view an unacceptable environmental cost - the abandonment will not in any way eliminate the demand for steel and aluminum. The best it can do is to keep it steady.
By use of a well known and sometimes industrial chemical reaction, the Boudouard reaction, I have actually come to believe that it might be possible to run aluminum plants (with their electrical demand coming from nuclear energy, as well as thermal energy for the reduction of carbon dioxide by thermochemical splitting) as carbon negative enterprise. I referred obliquely to this in my earlier post on this subject.
In that post I discussed the use of carbon anodes containing a fraction of biochar, wood thermally decomposed by heating in the absence of oxygen to make biocarbon that could be mixed into petroleum coke to reduce the carbon impact of aluminum production.
The paper cited at the outset of this post, by contrast, uses bio-oil to make carbon anodes.
Bio-oils are made by the destructive distillation of biomass in the absence of oxygen. Since biomass contains largely cellulosic polymers made up of chains of sugars and lignins, largely ether linked catecholic aromatics, bio oils tend to contain a fair amount of oxygen. Although they can be utilized in combustion, including combustion in engines, they tend not to be very stable. They oxidize to organic acids which not only burn poorly, but are prone to take up water as well as to be corrosive.
The current paper suggests a better use for biooils.
From the introduction to the paper:
Although proposed in the literature,(8) use of biomass char in electrodes is not performed commercially. According to laboratory studies,(9,10) partial replacement of petroleum coke by biomass char resulted in poorer anode properties. The anode density decreased, whereas anode resistivity and oxidation increased. This was attributed to low char bulk densities and to the presence of inorganic compounds (such as Na and K) which catalyze anode oxidation. While methods exist for removal of inorganic compounds from biomass char,(11) the low bulk density remains an issue. Pressurized pyrolysis, in a manner similar to ablative reactors, can increase the char bulk density,(12) but costs have yet to be determined. Furthermore, biomass char usually has an amorphous texture.(13) Anodes containing fillers with these textures have a high coefficient of thermal expansion (CTE),(14) making them susceptible to thermal shock cracking.(15) In anodes, isotropic coke can only be used as a blend component, but not exclusively.(16) It can be summarized that the poor performance of biomass char in anodes has several reasons: inorganic compounds present in biomass report to the char and during carbonization, the developing char does not pass through a liquid phase required for the development of the desired graphite-like, anisotropic texture...
The authors propose to use bio-oils prepared pyrolytically from several sources, guayle (creosote bushes), willow, switch grass, hardwood and - how ironic is this - horseshit, which they more politely euphemize as "horse litter."
Briefly, they heat their biomass in a fluidized sand bath at 500C in a stream of flowing nitrogen.
The oils distill out, and then are heated under argon at temperatures between 200 and 300 C, then "coked" in an oven at 900C.
It is found that the resulting cokes contain considerable elemental impurities. These can and do end up in the aluminum prepared in the cryolite electrolyzer utilized in the Hall Process, and can impact the quality of the aluminum.
Elements found in the biomass are calcium, vanadium, sodium, silicon and nickel at levels in the hundreds of parts per million, and zinc, manganese and titanium at concentrations an order of magnitude lower.
Potassium is also prominent in hardwood sources.
Horseshit contains large amounts of phosphorous, and the authors thus reject its use, since phosphorous content in anodes leads to higher electricity costs and in aluminum degrades its properties.
Alkali metals like potassium and calcium are said to increase the rate of oxidation of anodes and thus are undesirable, as is calcium - although calcium chloride is the working electrolyte in the FFC process for electrolytic titanium reduction (instead of croylite in the Hall process.)
Here's some pictures of the anode materials:

The caption:

The caption:

The caption:
Like the authors who produced the subject paper in my last post on this subject, these authors actually make electrodes that are only partially biological with respect to the carbon in the anodes.

The caption:
Although the authors here do not make a large enough sample to perform the standard tests for anodes used in the aluminum industry, the do some electrical measurements, such as "I V" curves, current vs. voltage:

The caption:
For some reason this graph lacks a key. It will probably show up in a "corrections" paper in this journal in the future.
The authors thus conclude:
The amount of aluminum produced by humanity is huge. I personally hope that this materials science effort will continue, since it is increasingly exigent to ban the use of dangerous fossil fuels and switch to nuclear energy.
This sort of thing offers some small hope for the future.
I wish you a pleasant day tomorrow.
Yet another cool allotrope of carbon.
The paper I'll discuss in this post is this one: Ultramicroporous Carbon Synthesis Using Lithium-Ion Effect in ZSM-5 Zeolite Template (Ko et al. Chem. Mater., 2018, 30 (18), pp 6513–6520)
When I was a kid people were taught that carbon had two allotropes, graphite and diamond. Perhaps some specialists were aware of Lonsdaleite, which may be thought of as a structural hybrid, part graphite and part diamond, an allotrope formed in meteorites on impact with the earth.
Today, many hundreds of allotropes of carbon are known, some of the more famous examples being buckminsterfullerene, C60, which as it turned out has always been present in lampblack but was not identified as such - the identification lead to a Nobel Prize - graphene, which is a graphite structure that is exactly one atom thick, and has been the subject of a huge amount of research and interest in possible applications, and single and multiple walled nanotubes, also the subject of much interest and some environmental concern.
The paper cited above reports a new one, and since my kid has been interested in templated polymeric materials, I was drawn to it.
It's "Ultramicroporous carbon."
Zeolites are well known nanostructured materials which are aluminosilicates. They occur naturally and are mined for their many useful properties, particularly where large surface areas are required, as in catalysis, carbon capture from flue gas, as well as in areas requiring separations by physics that is chromatographic in nature, depending on the diffusion into (and out of) the pores.
The authors here have used zeolites as templates for the construction of porous carbon, by utilizing the diffusion of lithium to guide acetylene into zeolite pores.
This introductory cartoon gives the general idea:

From the written introduction:
Similar to mesoporous silica, microporous zeolites have also been used as carbon templates. However, carbon synthesis using zeolite suffers from diffusion limitations for organic carbon precursors. Most zeolites have pore apertures of less than 0.9 nm in diameter, and this often leads to carbon deposition on the external surfaces of the zeolite particles.(14?21) The external carbon prevents the diffusion of the carbon source into the internal pores of the zeolite, resulting in a failure to replicate the entire pore system faithfully. To prevent external carbon deposition, the carbon precursor was fed as highly diluted in an inert gas flow, and the height of the zeolite bed in the carbon deposition reactor was minimized, preferably to a few millimeters. Nevertheless, when the carbon synthesis was scaled up to produce a zeolite bed greater than 1 cm in height, inhomogeneous carbon formation occurred between the upper and lower parts of the zeolite bed. As a strategy to solve this problem, Kim et al. incorporated La3+, Y3+, or Ca2+ ions into the zeolite template pores through a simple ion-exchange process.(22) These cations promoted the carbonization of ethylene or acetylene selectively inside the zeolite pores...
...We conjectured that ZTC synthesis using acetylene in Ca2+-ion-exchanged ZSM-5 zeolite might be improved if the Ca2+ ion (0.23 nm in diameter) is replaced by a smaller catalytic cation. We thereupon tested H+-ion-exchanged ZSM-5, but the result was poor. As a second attempt, we chose Li+ ion (0.18 nm in diameter), considering the high chemical affinity of Li+ ions with carbon to form graphite intercalation compounds, acetylides, and carbides.(30,31) With this assumption, we investigated the Li+ effect on ZSM-5-based ZTC synthesis via thermogravimetric analysis (TGA). The synthesized carbon product was characterized using transmission electron microscopy (TEM), scanning electron microscopy (SEM), Ar gas adsorption, powder X-ray diffraction (XRD) analysis, and electrical double-layer capacitance (EDLC) measurements. In particular, the EDLC characteristics were investigated to assess the recently reported effect of ultramicroporosity on anomalously increasing the capacitance.(32)...
The authors utilize a commercial zeolite, ZSM-11, the "11" referring to the ratio of silicon to aluminum. The ammonium in the commercial product was exchanged for lithium by treatment with a solution of lithium chloride.
They also investigated a number of other ions. This graphic shows the effects:

The caption:
Another graphic touches on these effects:

The caption:
They investigated several different gases as carbon sources, propylene, ethylene, acetylene and "bulky organic compounds" (unspecified). Only acetylene gave sufficient carbon deposition. The authors speculate that the low hydrogen to carbon ratio is responsible for this difference.
The resultant materials have controlled pore sizes:

The caption:
The resultant material shows remarkable electronic properties useful for capacitors and for electrodes. The authors map the electron density of the material.
This map is obtained from X-Ray Diffraction (XRD) and the application of software based calculations.
Here is the graphic reflecting the map:

The caption:
The materials show unusual "EDLC" (Electrical Double Layer Capacitance). The morphology of the material, allowing for the transport of metal and electrolyte ions is responsible for this effect, according to the authors and other workers. The relative drop in capacitance associated with higher current density is much smaller than for other materials.
Figure 7, in which "ZTC" stands for "Zeolite Templated Carbon":

The caption:
The summary:
...Furthermore, we confirmed that the ZSM-5 zeolite-templated carbon exhibited an anomalously high EDLC capacitance because of the presence of the ultramicropores.
Cool, I think. The electronic properties of carbon allotropes already play a huge role in technology, as I've discussed elsewhere in other posts, and to the extent that we are able to fix carbon under circumstances which have economic and technological importance - as opposed to the use of "carbon dumps" including but not limited to the planetary atmosphere - the more hope we have of slowing the atmosphere's destruction.
Have a pleasant Sunday afternoon.
Baby...baby...
Species Diversity and the Relationship to the Hydraulic Survivablity of Forests in Drought.
The scientific paper I will discuss in this post is this one: Hydraulic diversity of forests regulates ecosystem resilience during drought (Anderegg et al. Nature volume 561, pages 538–541 (2018).
Chronic international indifference to climate change - we are all involved, left and right, and in my opinion, all indifferent, all contemptuous of science that we don't intuitively like, based not on the facts of science and on the result of experiment, but on bias - has cause a remarkable rise in temperatures and macroscopic destabilization of the frequency of major weather extremes.
Among those extremes one that bothers me personally quite a bit - I'm an arbophile (if that's a word), a lover of trees - is drought. One of the things I love about the State of New Jersey, where I live, is its forests. As I watch trees die - something going on with increasing frequency - a little piece of me rots with the wood.
I am not a biologist or plant physiologist, but here and there, when time allows, I try to sneak in a few views of papers relating to the physiology of trees.
Some years back, on another site (where I was banned for telling the truth, not that telling the truth is something I would change about myself, even in the era of the revived Big Lie, for mere convenience or popularity) I wrote about one of these readings: Nitrogen, Climate Change, Drought, and Tree Physiology.
The Gods cursed Cassandra, but in all mythology, she is my moral favorite. The insistence among human beings to hear only what they want to hear is ageless.
Things have gotten much worse since I wrote that bit in 2010; when I wrote that piece the concentration of the dangerous fossil fuel waste in the planetary atmosphere was roughly 385 ppm. This month - near the annual minimum - the concentration is roughly 406 ppm.
Whatever it is we think we're doing isn't working and so long as we lie to ourselves by embracing popular nonsense thinking the acceleration of things getting worse will continue.
Anyway, brief excerpts from the Nature paper and some pictures:
Water, carbon and energy exchanges from the land surface strongly influence the atmosphere and climate; these exchanges are dominated by plants in most ecosystems1. Plant physiological responses to water stress influence these fluxes5,6, and the resulting land-surface feedback effects influence local weather as well as the regional atmospheric circulation7. Furthermore, changes in vegetation physiology and cover can drive shifts in sensible and latent heat fluxes that intensify droughts2,3,4,8. Anthropogenic climate change is expected to intensify the hydrological cycle globally, leading to more frequent and more severe droughts in many regions9. Therefore, understanding the drivers of land–atmosphere feedback effects during drought and simulating them in Earth system models is critical for robust future projections and assessment of climate change impacts.
Seminal work has shown that grassland plots with more species exhibit smaller declines in productivity during drought and recover productivity much faster following drought10, indicating that plant biodiversity—particularly functional diversity—may be important for capturing how the land surface interacts with the atmosphere during extreme events. Indeed, it is well-established that—just as a diversified stock portfolio is more likely to survive market turbulence11—diversity can stabilize community function through multiple mechanisms12. First, diverse communities are more likely to contain species with different traits that dictate how they respond to disturbances13. As a result, at least some species are likely to persist through any given disturbance14. Second, diverse communities are more likely to contain competitors that exhibit compensatory dynamics: when drought causes one species to decline in function, its competitor may increase in function and stabilize community function12. Critically, diversity–stability effects are mostly absent in most global land-surface models, most of which represent each biome or plant functional type with a single set of functional traits15, partly owing to a lack of understanding of which functional traits are the most important at ecosystem scales.
In their study of forests, the authors consider a number of factors, the age of the forest, leaf area, and several factors related to the biophysics of water transport, the point at which 50% of the xylem water conductivity is lost, and a factor they call "HSM," the "hydraulic safety margin," by considering the species which make up 80% of the biomass of the forest, and physical traits like wood density.
This graphic gives an idea of their focus:
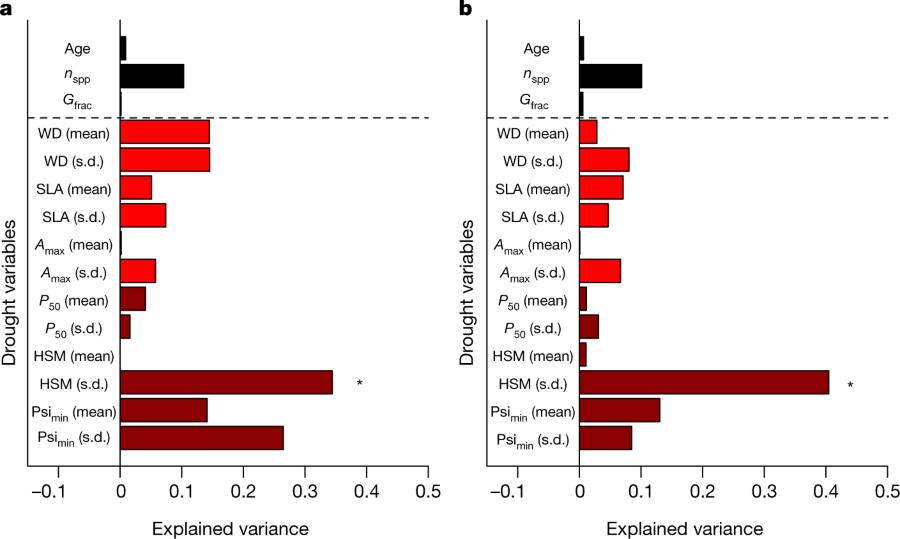
The caption:
Based on statistical inference, they conclude that the HSM factor is important.
They write:
Another graphic on the topic:
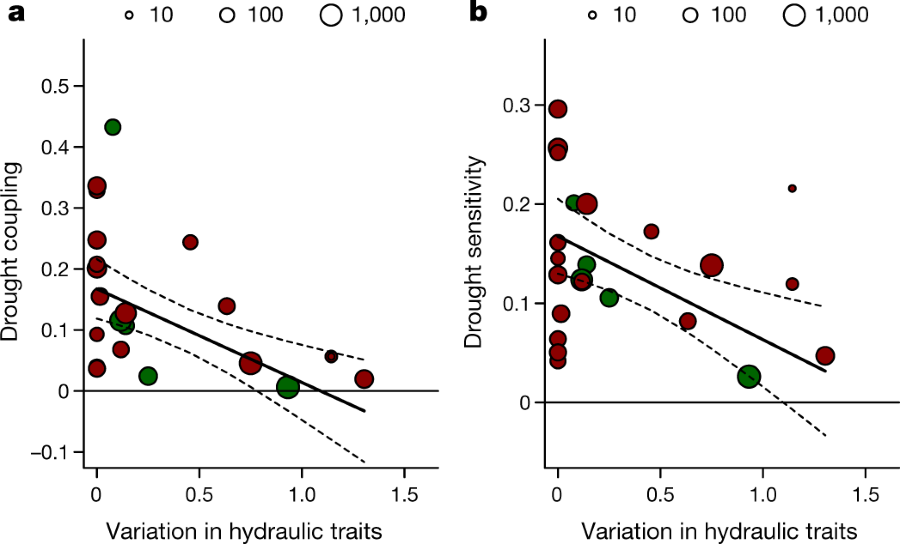
The caption:
They do some work that they define as "preliminary" using satellite data to study the water health of forests, focusing in particular on the United States - a prominent North American country ruled by an ignorant racist with a fondness for sexual assault and his equally mindless enabling cranks - but also encompassing global "estimates."
They produce this graphic:
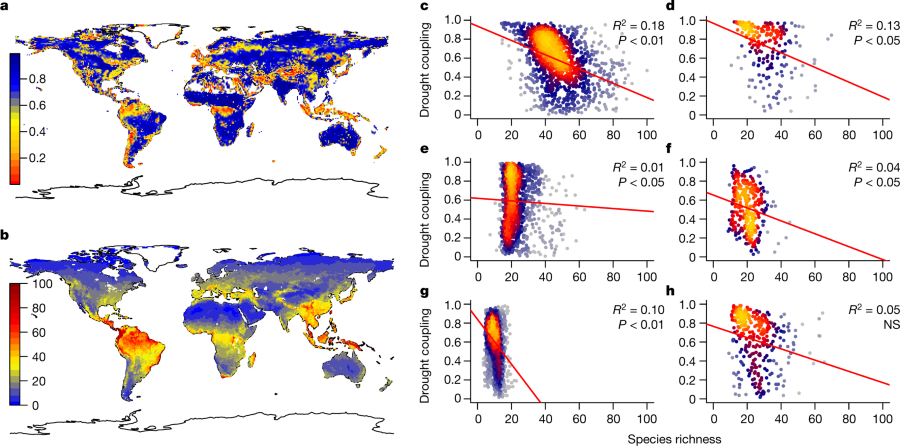
The caption:
They find, unsurprisingly but nevertheless disturbing in the age of monoculture, that the best resilience in forests facing drought is species diversity. (I'm not sure, however, that adding diversity is a good idea, since the effects of introduced species both intentional and unintentional has greatly impacted the natural biosphere.)
They have a nice and detailed description of their methods in the paper. In conclusion to their discussion they write:
A nice read.
If, by the way, you are interested in supporting and learning science, it does appear that Nature is offering a significant discount on a subscription making it quite affordable. My wife bought me one for my birthday, which saves my fat ass from traveling to scientific libraries a bit.
I love growing old with her, but as I near the end of my life, which I have loved so much, I am pained at what we are leaving behind.
I trust you're having a great autumn weekend.
Recovery of Phosphorous from the Hydrothermal Gasification of Sewage Sludge.
The scientific paper I'll discuss in this post is this one: Phosphorus Transformation in Hydrothermal Pretreatment and Steam Gasification of Sewage Sludge Feng et al Energy Fuels, 2018, 32 (8), pp 8545–8551. The text, which I will quote verbatim without the use of the parenthetical (sic) notation, shows the linguistic seams of having been translated from Chinese into English, but the ideas are far more important than perfectly grammatical English.
I have read somewhere - it may have been Vaclav Smil's outstanding rumination on nitrogen fixation, Enriching the Earth - that the main reason that China agreed to meet with Henry Kissinger, and then Nixon, was not to achieve peace but was rather concerned with the practical need to have access to American Haber-Bosch technology for the fixation of nitrogen. 1972 was, after all, only 23 years after Mao's victory over the nationalists, and coupled with the disaster of the cultural revolution, the ideological attacks on intellectuals, and the continuous strain on China's soils, it was not clear that China could feed its massive population, and it may not have been clear to the Chinese leadership that they could survive a massive famine associated with the total collapse of agriculture.
The words "green revolution" have come to mean something quite different in the 21st century than what they meant in the mid-20th. From my perspective the 21st century meaning consists largely of a specious lie we tell ourselves to avoid reality, but in the mid 20th century the meaning was very much connected with the growth of a major technology connected with farming: the agrochemical fertilization of soils and (like or not) the development of chemical pest control, including, but hardly limited to, the control of weeds.
Without these developments, Thomas Malthus would not be quite so forgotten nor quite the subject of intellectual bemusement he seems to have become, if he is considered at all by anyone other than old people like me.
It wasn't all that long ago that in many places it was impossible to feed huge swathes of the world's population
In 1943, in the Bengal, between 2 and 3 million people starved to death; for perspective, this is roughly 7 to 10 times the number of British combat deaths in all of World War II.
Worse, during the period of Mao's "Great Leap Forward" (1958-1961) - the numbers are controversial and will probably never be accurately known, it is estimated that tens of millions of people, maybe as many as 40 million, starved to death. While Mao and Zhou Enlai politically survived the "Great Leap Forward," according to the account I read, it wasn't clear to them that they could survive a second round. (Effectively their style of Goverment didn't survive them; if China is a socialist state, it is comparatively an ersatz version of what Mao was trying to create.)
Nixon, the way I heard it in such a way as to believe it, could open the door to technology transfer to China of fertilizer production (and other technologies) so China opened the door to Nixon.
A desire for peace had nothing to do with it. (Nixon's war like purpose was to further isolate the Soviet Union.)
I will not discuss chemical pesticides here further, nor will I talk any further about nitrogen fixation, a necessary component for there to have been an agricultural "green revolution" spreading rapidly in the 1950's, but will instead point to the cited paper's discussion of a critical agricultural commodity that is far more subject to depletion than fixed nitrogen is, phosphorous.
From the introduction of the paper:
Despite producing high-quality hydrogen-rich syngas, as a thermochemical disposal method, gasification results in a highly concentrated phosphorus (P) content in sludge ash.(7) P is an essential element for all living organisms. Phosphate rock is the primary source of P fertilizer, while it will be depleted in 1 century.(8) In National Mineral Resource Planning issued by the Ministry of Natural Resources in China (2016–2020), P was listed in the category of strategic minerals. SS produced from water treatment is rich in P. In China, there was approximate 125 000 tons of P of 24.186 million tons of SS production in 2012.(9) Furthermore, the P concentration in sludge is still increasing with the improvement of the water treatment technology.(10) Therefore, it is promising to develop P recovery technology from SS, which is recognized as the second largest source of P.(7)
Gasification need not be, even if sewage sludge contains considerable carbon, be carbon negative, nor carbon neutral. Au contraire it can be a source of carbon pollution inasmuch as gasification requires considerable heat.
In the author's work, they treat sewage sludge from the Shanghai municipal sewage treatment plant, at a range of 4 temperatures between 200C and 260C - subcritical water/steam - for a half hour, with the extrema being performed both in the absence and presence of 10% calcium oxide.
Following this treatment the resulting hydrochars were gasified with steam at 900C.
The carbon impact of this treatment is thus entirely dependent on the source of heat. If the heat source is coal, this process will be from a carbon perspective, disastrous. Were the heat sourced from nuclear energy - and it wasn't here, although China has built advanced high temperature research nuclear reactors - the process can be carbon negative, depending on how the resulting carbon originating from the sludge is used.
In any case, the problem with the gasification of sewage sludge and other biomass sources is very much connected with the fate of various other inorganic constituents, notably sodium and potassium, but silicon and metals can also be problematic. Depending on conditions, sulfur can be oxidized to species resulting in sulfuric acid, nitrogen to nitric acid, although it is also possible to transform these elements - under other conditions - to reduced species such as sulfides, sulfur, ammonia and organic amines.
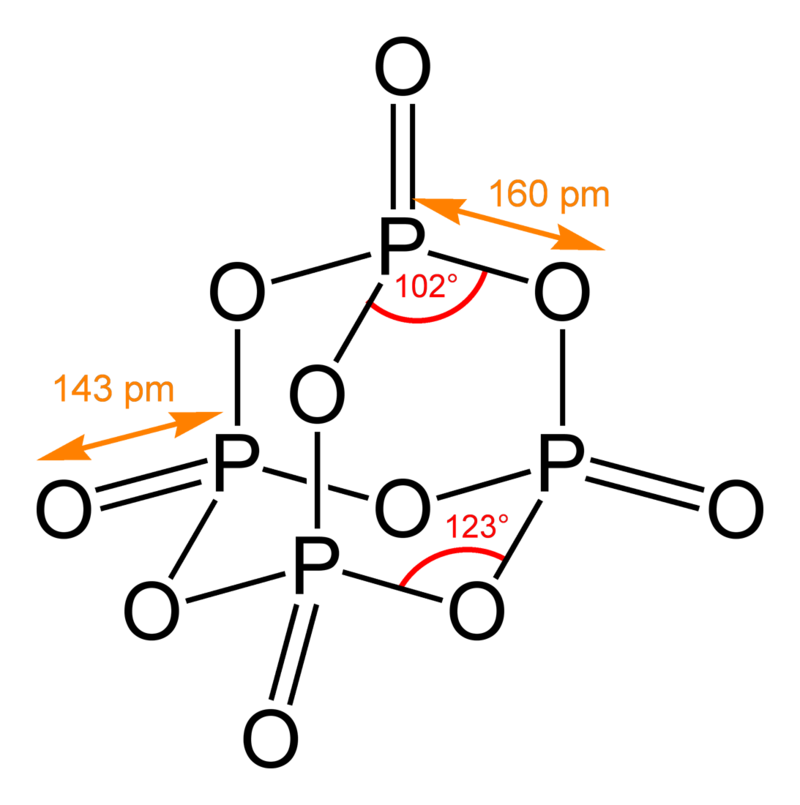
Phosphorous can also be corrosive under the right conditions and hence this work. Phosphorous speciation is reported to consist of inorganic and organic phosphorous compounds. Among the inorganic phosphorous species are phosphoric acid, phosphorous pentoxide (P2O5) and phosphates of aluminum, manganese, and iron, as well as calcium phosphate where calcium oxide was added. Phosphorous pentoxide can volatilize at high enough temperatures; in this form it is an allotrope that is a dimer, P4O10 with an adamantane type structure having alternate oxygens and phosphorous atoms, with each phosphorous bound to a doubly bonded oxygen external to the ring structure:
Some graphics from the paper relating to the recovery of phosphorous from sewage sludge:

The caption:

The caption:
The effect of temperature on the volatilization of phosphorous:

The caption:
Some graphical representation of speciation in the presence of CaO additions:

The caption:
Some of the authors' concluding remarks:
My personal sense of disgrace is not that so much that I live in a country increasingly ruled by sybaritic mindless pigs - although this is troublesome - but is more connected with the obvious fact that we do not care a wit for future generations; our contempt for them is not even barely disguised.
(I want, I need a Tesla car!!!!)
At least these scientists are leaving them with some important information; one wishes one could feel that they will have the resources to employ this information not just for survival, but in order to have the ability to do the good and great things that properly treated, women and men can do.
Have a nice weekend.
Let me see if I can clear something up for you.
A very simple fact is that to determine the capacity utilization of any energy system over a period of time, you take the rated capacity in units of watts, and multiply it by the period of time being studied in units of seconds.
A sixth grader should know this much physics: A Watt = Joule/second, 1 W = 1 J/s
So, if I go to the data table on the master register of wind turbines in Denmark (accessed 9/27/2018) and use simple Excel operations to sum column C, I can find that all of the operating wind turbines in Denmark, all 6211 of these soon to rot pieces of shit, I find that the total rated capacity is 5,717,454 kW, or 5,717,454,000 W.
Now I can go over to the most recent completed column, that of August 2018 - which is column BM in the very same spreadsheet - I can find out how much energy all 6,211 of the Danish bird and bat grinders produced in that month, in units of kilowatt-hours. The Danes have happily provided this number in their spreadsheet at the bottom, so one doesn't even if one is, say a dumb guy in Greenpeace who can't use the "sum" function in Excel, he can still see that the sum for the entire month of August 2018 was 940,024,330 kwh.
One can convert kWh to Joules by multiplying by 3600 (the number of seconds in an hour) and 1000 (for the "kilo" ) and find out that the all 6,211 greasy Danish wind turbines produced 3.380488 petajoules (peta = 10^15) on a planet where humanity consumed (as of 2016) 587 exajoules (exa = 10^18) in that year.
Now, this may be hard for a person who has been unable to grasp what it means to all future generations that more than a trillion dollars has been squandered on the wind industry in the last ten years with the result that the concentration of carbon dioxide has risen, as of September 2018 by 23.32 ppm over September of 2008, but using these numbers, and simple arithmetic, one can calculate what the capacity utilization of all of Denmark's wind turbines were in August 2018.
And unless there are a whole bunch of fucking Danes running around changing the blades every time the wind speed changes, remarks about the size of the blades could do if they were a different size don't matter a fucking wit, even though the Danes do list the turbine blade sizes in column D in their spreadsheet.
The month of August is 31 days long. A day has 24 hours in it, and each hour has 3600 seconds. By multiplication, this means that the month of August has 31 X 24 X 3600 = 2,678,400 seconds.
Again, the rated capacity of any power plant, a nuclear plant, a gas plant, a coal plant, a wind plant, a solar plant is its peak rating times the number of seconds in the period being discussed. So the 6,211 wind turbines in Denmark, with a capacity of 5,717,454,000 W for a period of 2,678,400 seconds should produce 15.31363 Petajoules if they operated at 100% capacity utilization.)
(As a practical matter, only nuclear plants actually operate at 100% capacity utilization for extended periods of time: The capacity of US nuclear plants can be found here. Overall in 2018 US nuclear power plants had a capacity utilization of 92.2%, with 23 of them operating at 100% or greater than their rated capacity.)
Now - I hope this isn't too hard - we calculated that the actual energy produced by the 6,211 Danish wind turbines that a future generation will have to disassemble (or watch rot) in about 30 years, with the largest percentage falling into "decommissioned" garbage in 20 years, actually produced 3.380488 Petajoules. So if we divide 3.380488 by 15.31363 and multiply by 100 to make that magic "percent" word that the people who bet the future of humanity on so called "renewable energy" so love, we find that the 6,211 bird and bat grinders in Denmark had a capacity utilization of 22.0985%
We can also find the average continuous power for all 6,211 wastes of concrete, aluminum, steel, copper and polymers was in August 2018: We divide the number of joules produced in August of 2018 by the number of seconds in August to find that the average continuous power was 1,263,473,561 kw or 1,263.474 Megawatts.
This is remarkably close to the average power rating of a single reactor in Pennsylvania, the TalenEnergy Susquehanna Nuclear Power Plant, a reactor which produced 100.3% of its 1,298 MW capacity for all of 2018 up to July, the last month listed in the spreadsheet linked above.
It produced that much energy in a single small building, as opposed to a whole fucking European country:

Here's a picture of the reactor core where all that energy was produced:

It is the extremely high energy to mass ratio that makes nuclear energy cleaner than all other options.
Thanks for the smug lecture on wind turbine blades. It is, of course, as one might expect when one is going to suffer through defense of this indefensible crap, meaningless. Convoluted and tortured defenses like these may have something to do with the fact that despite all of the bullshit defenses of this unconscionable attack on all future generations - betting the planetary atmosphere with on so called "renewable energy" with Kavanaughesque drunken recklessness - the concentration of the dangerous fossil fuel waste was, for the week beginning September 16, 2018, 405.69 ppm, whereas just ten years ago, and a trillion bucks of "wind investment" ago, it was 383.32.
It can be shown by anyone who gives a shit about humanity - anyone who cares a wit and keeps track of these things - that 23.32 ppm in a ten year period, 2.33 ppm/year, is the fastest rate of atmospheric degradation ever recorded going back to the late 1950's, in any decade.
Facts matter.
And it is a fact that people don't run up to wind turbines and change the blades when the wind speed changes.
The wind industry is not working. It has never worked. It won't work, if "working" means arresting climate change, something that environmentalists care about, if not people for calling for turning every damned mountaintop to a wind industrial park for their stupid fantasies about Tesla electric cars and other consumer electronic crap.
Heckuva job wind freaks! Heckuva job! You must be very, very, very, very, very proud.
I wish you a pleasant Friday.
A Very Cool Rumination on Vapor-Liquid Cubic Equations of State.
The paper to which I'll refer here, written by scientists at Institute of Process System Engineering, Qingdao University of Science & Technology, People’s Republic of China is this one: Research into the Polynomial Alpha Function for the Cubic Equation of State (Wenying Zhao , Xiaoyan Sun, Li Xia, and Shuguang Xiang, Ind. Eng. Chem. Res., 2018, 57 (38), pp 12602–12623)
When I was a kid, I was doing a bunch of hydrogenations and because this sort of thing can be a little boring, I decided to program my HP41C calculator to solve cubic equations for the Van der Waals. The traditional way to monitor these things was to wait until the reaction stopped taking up hydrogen and work it up, but I was a dorky kid and so I programmed the calculator to do it. If I recall correctly, it came in within 2 or 3 percent of the theoretical value, so it kind of worked, but was more or less useless, because the reaction worked just as well if you didn't calculate the number of moles of hydrogen uptake from the P,V,T data.
Before my son went off to college, I challenged him to do the same thing (using Mathematica) for the Peng Robinson cubic equation, and he sort of made a decent stab at it, but largely blew it off to spend the last free days with his high school friends.
More and more, as I contemplate the awful state of our environment with respect to carbon dioxide (and other gases), I fantasize about reactors with mixtures of gases in them at high temperature - often involving supercritical fluids, especially the strange supercritical state of water - and I find myself thinking how I should find the time to think more about equations of states and their refinements.
It was thus with some joy that I came across the interesting paper linked herein. Here's a graphic from the paper:

The intro:
Formally, alpha functions can be divided into polynomial and exponential functions. In this work, we review and analyze the functional forms, parameter correlations, and scope of the polynomial alpha functions developed over the past 40 years. The research methods and developing trend for this kind of function are also reported. A review of exponential alpha functions will be made in a separate publication.
The "evolutionary tree" of cubic equations of state:

I suppose only a dork could be filled with joy at this sort of thing, but I'm a dork, and damned proud of it too.
I have to find some time to play with this paper.
I'd send it to my son, but he, apparently, has a life.
I'd just thought I'd throw this out there, because I think it beautiful, and more than ever, we need beautiful things.
I just realized the paper is open sourced if you'd like to try it.
Temperature Dependence of Biochar Hardness, a Factor in Displacing Coal Based Coke in Steel Making.
The paper I'll discuss in this brief post is this one: Dynamic Hardness of Charcoal Varies According to the Final Temperature of Carbonization (Hein et al Energy Fuels, 2018, 32 (9), pp 9659–9665)
I am a critic of the so called "renewable energy" industry. My main argument is that it has proved demonstrably ineffective at climate change. At the outset of this century it was decided to "invest" trillions of dollars in this pixilated scheme - this on a planet where billions of people lack access to electricity, clean water, and basic sanitation - with the result that the rate of accumulation of the dangerous fossil fuel waste carbon dioxide has accelerated, not decelerated. The fastest growing source of energy - as measured in new exajoules of production - in this century has been dangerous fossil fuels, the increase lead by coal, which increased by 60 exajoules as compared to the nearly 130 exajoules of additional dangerous fossil fuel combustion added to the world energy equation since the year 2000.
Another criticism I have of the so called "renewable energy" industry is that it is not really "renewable." As I pointed out in a recent post in this space on the carbon cost of production of metals, the metal intensity of so called renewable energy dwarfs the requirements of the much cleaner source of energy, nuclear energy.
I quoted this paper: Metals for a Low Carbon Society (Vidal et al Nature Geoscience volume 6, pages 894–896 (2013):
... If the contribution from wind turbines and solar energy to global energy production is to rise from the current 400 TWh (ref. 2) to 12,000 TWh in 2035 and 25,000 TWh in 2050, as projected by the World Wide Fund for Nature (WWF)7, about 3,200 million tonnes of steel, 310 million tonnes of aluminium and 40 million tonnes of copper will be required to build the latest generations of wind and solar facilities (Fig. 2). This corresponds to a 5 to 18% annual increase in the global production of these metals for the next 40 years. This rise in production will be added to the accelerating global demand for ferrous, base and minor metals, from both developing and developed countries, which inflates currently by about 5% per year5,6..
Although, as I showed in the post, aluminum is dependent upon petroleum coke and steel on traditional coke from coal, I am always interested in alternatives, to the extent to which they can be realized.
That is the subject of the cited paper. (Historically steel was made using wood as a carbon source, albeit on a planet with a small fraction of its current population, and certainly not a population "investing" in bat and bird grinding wind turbines that turn to landfill every 20 years or so.)
From the introduction:
Brazil has been pointed out as the only country that industrially uses charcoal as a source of carbon monoxide and heat in blast furnaces for steel production.(5) The main consumers of the charcoal are pig iron, steel, and iron alloy industries and, to a lesser extent, trade and residential consumers.(3) According to Vieira et al.(6) one of the problems faced by the Brazilian steel industry is the heterogeneity of the charcoal used in the steel fabrication with reference to physical, mechanical, and chemical properties and the low yield in the carbonization processes currently used.
Variations in wood and carbonization processes led to variations in the gravimetric yield and charcoal quality.(7?9) High wood density, lignin content, and low ash content are characteristics that may be considered as wood quality indices for charcoal production.(10,11) With regard to the quality of charcoal, a higher fixed carbon (FC) content and lower ash and volatile contents are associated with a high lignin content and low holocellulose and extractive contents in wood.(10)...
...Charcoal has two main functions in the blast furnace, providing energy for the process in the form of heat and being the reducing agent of iron ore,(1) but also the charcoal layers must mechanically withstand the weight of the iron ore inside the blast furnaces.(14) According to Assis et al.,(8) the mechanical characteristics of charcoal are also controlled by the characteristics of precursor raw material, for instance, specific gravity, moisture, chemical composition, and anatomical features, and also by the two key carbonization settings: final temperature and heating rate.
To our knowledge, there is no standardized method for evaluating strength characteristics of vegetable charcoal. Traditional methods to evaluate the mechanical behavior of charcoal are neither precise nor repeatable. Among the mechanical properties of the charcoal, the friability has been determined by means of the drum test.(15) To carry out the mechanical characterization in the charcoal sample, 500 g of material is placed in a fixed rotating drum that rotates at 30 rpm around a horizontal axis, 30 cm in diameter and 25 cm in length.
The authors examine several different species of trees as a source of charcoal, as well as the effect of temperature and other conditions on the preparation of chars.
Here's a picture of their hardness tester and some charcoal:

The caption:
DH = dynamic hardness.
A graph of some findings:

The caption:
The relationship between the density of wood and the hardness:

The caption:

The caption:
And the density of the charcoal as a function of the temperature of pyrolysis:

The caption:
The relationship between charcoal yield and hardness:

The caption:
The authors claim that the species of wood doesn't matter quite as much as the temperature of pyrolysis.
The steel industry - even without investing heavily in stupid seabird and bat grinders to make small amounts of electricity while requiring increasing reliance on the dangerous fossil fuel natural gas - currently consumes over one billion tons of coal based coke per year.
I personally don't endorse grinding up two billion tons of trees each year to make steel, but that's just me, since I seem to be in the minority, since I know that so called "renewable energy" not only hasn't worked and isn't working, but that it won't work.
No one now living will ever see an atmospheric carbon dioxide concentration lower than 400 ppm again, mostly because left and right, we are invested in lying to ourselves.
I believe that carbon for steel making can be (as in "technically feasible" as opposed to "politically feasible" ) derived from the thermochemical reduction of carbon dioxide to the monoxide, and, to the extent it may prove necessary, disproportionation of carbon monoxide into carbon powder and carbon dioxide (which will be recycled) using Boudouard chemistry.
To the extent that said carbon dioxide is removed from the atmosphere (via seawater) and to the extent steel has a carbon content, this is effectively sequestered carbon.
The heat to drive this reaction can only come from nuclear energy in a clean world, as opposed to the delusional world in which we live in which nuclear energy is demonized by people who can't think and who thrive on fear and ignorance.
Have a nice day tomorrow.
Removal of the Ammonium Cation From Waste Water Using Ion Selective Battery Electrodes.
The paper I will discuss in this post is this one: Ammonium Removal from Domestic Wastewater Using Selective Battery Electrodes (Logan et al Environ. Sci. Technol. Lett., 2018, 5 (9), pp 578–583
One of the things that really freaks me out as I contemplate what we have done in my generation to screw over all future generations is the situation with respect to fixed nitrogen.
Specifically in my rants and rages, I am concerned with the accumulation of growing levels nitrous oxide - "laughing gas" that isn't funny - in the atmosphere where it acts as a greenhouse gas but, of more concern, as an ozone depleting agent. Nitrous oxide, N2O, is of course, a part of the normal nitrogen cycle, which has operated for billions of years, but the invention of Haber-Bosch process in the early 20th century severely impacted the established balance while simultaneously preventing world wide starvation and making it possible for people without access to salt peter mines to make gun powder for their much loved wars. (Germany would have lost World War I in 1915 were it not for the Haber Process.)
I suspect that nitrous oxide will be the major ozone depletion agent by the end of the 21st century should there be an end to the 21st century. It is not easy to ban - actually it's impossible to ban - because the world food supply does, and always will, depend on access to nitrate and ammonia fertilizers.
One of the other effects of excess nitrogen is to destroy rivers and lakes and - as we are seeing in Florida now in part because of "Red Tide Rick's" traditional Republican hatred of anything involving saving the environment - large stretches of ocean with eutrophication, a burst of growth of microorganisms which die and consume all the oxygen in the water as they decompose, thus killing any aquatic creature that needs, um, oxygen.
It is therefore with great interest that I read the paper cited at the outset of this post.
The introduction covers the problem pretty well and reviews the proposed approaches and problems with those approaches to dealing with this very serious problem:
Their approach arises from a consideration of a type of electrode that has been developed specifically for batteries, a "Prussian Blue" electrode comprised of copperhexacyanoferrate, which they noted, involves the capture and release of different cations utilized in electrolytes at different voltages. They make one of these electrodes under fairly mild conditions - it is supported on a carbon cloth - and then run some tests, beginning with some laboratory solutions of common salts found in wastewater in the presence of ammonium salts, and then with "synthetic wastewater" and finally with real wastewater.
Here's a cartoon describing their system along with some bar graphs showing the selectivity of this system toward the ammonium ion as compared to sodium:

The caption:
This graphic shows other results for the removal of cations from waste water.

The caption:
They offer their conclusions:
...and then - as honest people do - some caveats and limitations...
With... ...technological advancements, our approach could represent an effective method for the selective removal of ammonium from various waters, including domestic wastewater, with low energy consumption.
Nice, I think.
I trust that you are having a pleasant Sunday afternoon.
Egypt Brings New Gas and Wind Plants on Line.
This news item is here: Egypt Brings New Natural Gas and Wind Power Plants Online
An excerpt:
And of course, the lipstick on the pig, the wind plant:
The usual dishonest technique of reporting the dangerous fossil fuel plant - which despite being a dangerous plant is fully capable of running continuusly - and the wind plant in terms of power and not energy operates here.
Around the world, wind turbines typically operate with a capacity utilization of between 30% and 40%, with the higher figure being rather unusual.
One can show, by appeal to the Master Register of Danish Wind turbines that the capacity utilization of all commissioned wind turbines that their capacity utilization is typically in the high twenties for the nation as a whole.
Thus the 580 "MW" wind plant, at 30% capacity utilization will be the equivalent of a 174 MW gas plant, and at 40% - which I personally doubt given where it's located - a 232 MW gas plant. And of course, the wind plant needs the gas plant to be worth anything, since it is generally recognized among all but the most delusional people in the world, that the wind sometimes does not blow.
The lifetime of Danish wind turbines averages less than 18 years, and they're not located among sand dunes as the Egyptian wind plant is:

One does not need a degree in mechanical engineering to have some appreciation of the fact that blowing sand is not good for things with moving parts exposed to the elements.
Egypt's government says it wants to produce 20% of its electricity by so called "renewable energy" with the usual "by such and such a date" statement, "such and such a date" in this case being 2035, 17 years from now. By that time their new wind plant will most likely be sand blasted inoperative junk waiting to be disassembled for metals scavengers who will face the toxicity of the stuff therein and the grease cheerfully, because that's what impoverished people do, suffer for our bourgeois sins.
Egypts new capacity, accounting for the huge difference in capacity utilization between wind and gas plants is on the order of 5%, at least for as long as the wind plant lasts. It is worth noting that the restart of a gas plant because the wind is blowing wastes gas. It's probably more economic to simply run the gas plant full bore without shut down whether the wind is blowing across the sands or not.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512