NNadir
NNadir's JournalExperimental Determination of the Bare Sphere Critical Mass of Neptunium-237.
The paper I'll discuss in this post is this one: Criticality of a 237Np Sphere (Rene Sanchez et al., Nuclear Science and Engineering, Nuclear Science and Engineering, 158:1, 1-14 (2008)).
Neptunium is the only actinide element that is easy to obtain in an isotopically pure form simply by chemically isolating it. This is because all of the isotopes except Np-237, which has a half-life of 2,144,000 years, that are known and which form readily in thermal spectrum nuclear reactors - which represent almost all of the world's commercial nuclear reactors - are short lived. The half-life of Np-238, the parent of plutonium-238 is 2.117 days, and the half-life of Np-239, the parent of plutonium-239 is 2.356 days. Thus even in a continuous on line isolation system from a critical nuclear fluid of the types now under discussion, chiefly molten salt type reactors, any isolated neptunium would decay, with a few weeks time to essentially pure Np-237.
Neptunium is routinely formed in the operation of commercial nuclear reactors. In thermal reactors, neptunium has a high neutron capture cross section and its fission is rare. Chiefly it is transmuted into plutonium-238, the accumulation of which has the happy result, in high enough concentrations (albeit not necessarily routinely formed concentrations), to make reactor grade plutonium that is essentially unusable in nuclear weapons. (As a practical matter, it is much easier to make nuclear weapons from natural uranium by separating the U-235 than it is to make it from reactor grade plutonium, and since it is impossible for humanity to consume all of the natural uranium on the planet, it will never be possible to make nuclear war impossible.)
In a fast neutron nuclear spectrum, neptunium can form a critical mass, and thus can be utilized as a nuclear fuel (or in theory, a nuclear weapon).
I personally favor fast spectrum nuclear reactors, since they represent the potential to ban all energy related mining, dangerous natural gas wells, fracked and "normal," dangerous petroleum wells, fracked and "normal," all the world's coal mines, and in fact, all of the world's uranium mines for many centuries to come, utilizing the uranium already mined and the thorium already dumped by the lanthanide industry.
The so called "minor actinides," generally including neptunium, americium, curium and sometimes berkelium and californium, all have useful properties; there has been a lot of discussion in the scientific literature of using neptunium and americium as constituents of nuclear fuels, to eliminate the often discussed, but entirely unnecessary waste dumps for the components of used nuclear fuel.
From the introduction of the paper:
Neptunium-237 is a byproduct of power production in nuclear reactors. It is primarily produced by successive neutron captures in 235U or through the n, 2n reaction in 238U. These nuclear reactions lead to the production of 237U, which decays by beta emission into 237Np (Equation 1):

It is estimated that a typical 1000-MW electric reactor produces on the order of 12 to 13 kg/yr of neptunium.2 Some of this neptunium in irradiated fuel elements has been separated and is presently stored in containers in a liquid form. This method of storage is quite adequate because the fission cross section for 237Np at thermal energies is quite low, and any moderation of the neutron population by diluting the configurations with water would increase the critical mass to infinity. However, for long-term storage, the neptunium liquid solutions must be converted into oxides and metals because these forms are less movable and less likely to leak out of containers.
As noted in Ref. 3, metals and oxides made out of neptunium have finite critical masses, but there is a great uncertainty about these values because of the lack of experimental criticality data. Knowing precisely the critical mass of neptunium not only will help to validate mass storage limits and optimize storage configurations for safe disposition of these materials but will also save thousands of dollars in transportation and disposition costs.
The experimental results presented in this paper establish the critical masses of neptunium surrounded with highly enriched uranium (HEU) and reflected by various reflectors. The primary purpose of these experiments is to provide criticality data that will be used to validate models in support of decommissioning activities at the Savannah River plant and establish welldefined subcritical-mass limits that can be used in the transportation of these materials to other U.S. Department of Energy facilities. Finally, a critical experiment using an a-phase plutonium sphere surrounded with similar HEU shells and using the same setup used for the neptunium experiments was performed to validate plutonium and uranium cross-section data.
A brief excerpt of the materials utilized in these experiments:
…The analysis showed that the sphere was 98.8 wt% neptunium, 0.035 wt% uranium, and 0.0355 wt% plutonium. There were also traces of americium in the sphere. Table I shows the elements found in the chemical analysis of the sprue. Approximately 1% of the mass of the sphere was missing because the sprue sample did not dissolve completely.
To reduce the gamma-radiation exposure to workers, which comes mostly from the 310-keV gamma ray from the first daughter of 237Np, 233Pa, the neptunium sphere was clad with a 0.261-cm-thick layer of tungsten and two 0.191-cm-thick layers of nickel. The gamma radiation at contact with the bare sphere was reduced from 2 R/h to 300mR/h for the shielded sphere. Table II shows the dimensions, weights, and calculated densities of the neptunium sphere and different cladding materials. The total weight of the sphere, including cladding materials, was 8026.9 g. Figure 2 illustrates how the neptunium sphere was encapsulated. Except for the tungsten layer, both of the nickel-clad materials were electronbeam welded. In addition, a leak test was conducted for the nickel-clad layers to ensure that the neptunium metal and possibly some neptunium oxide produced in the event of a leak were contained within these materials and not released into the room or the environment.
Table 1:

This is a highly technical paper, and it is probably not of any value here to excerpt all that much of it. Nevertheless, there is a great deal of public mysticism about nuclear technology, mysticism that is killing the world, since nuclear energy is the only technology that might work to ameliorate, stop, or even reverse climate change. There is so much mysticism and misinformation that completely scientifically illiterate morons like say, Harvey Wasserman, can find people ignorant enough to believe he is, in fact, an "expert" on nuclear issues. (He's not. He is an abysmally ignorant fool, whose ignorance is killing people right now.)
With this in mind, I thought it might be useful to show some diagrams and photographs of the work that was performed here and that is found in the original paper:







A student of nuclear history will recognize that these experiments are very much like the experiments with the "demon core" that killed the nuclear weapons scientists Harry Daghlian and Louis Slotin in separate experiments in 1946. The remote equipment here is obviously designed to prevent that sort of accident from recurring.
The authors explored a number of different systems and reflectors, including both polyethylene and steel. In the process of conducting these studies, they refined some nuclear data on uranium isotopes, a valuable outcome.
From their conclusion:
Currently the main use for Np-237 is as a precursor for Pu-238 for use in deep space missions. Production of this important isotope has resumed at Oak Ridge National Laboratory, albeit on a small scale.
If we are interested in saving the world - there isn't much evidence that we are - neptunium can play a larger role in doing so, and thus this historical work is of considerable value.
A related minor actinide, which is also a potential source of Pu-238, although this plutonium will always be contaminated with Pu-242 owing to the branching ratio of the intermediate Curium-242, is americium-241.
It was estimated, in 2007, that the world inventory of these valuable elements was, as of 2005, was about 70 tons of Np-237, and 110 tons of Americium. It is desirable, critical actually (excuse the pun) that these materials be put to use.
I wish you a pleasant Sunday.
Why I switched my support here, not that it matters, from Warren to Yang.
I love Elizabeth Warren, because my feeling is that she has a flexible mind; if nothing else this will be a critical feature that a future President must have if we are to save anything from this unnatural disaster represented by the ignorant pig in the White House.
She also has a real chance to be the nominee, and if she is, I will be thrilled to vote for her.
Nevertheless, my sons prevailed on me to take a look at Andrew Yang, and to the extent I have time to engage in politics, I did so.
What I think doesn't matter, actually. I am not that politically engaged and to the extent I am, I'm strictly "anyone but Bernie" in the Primaries, and, in the general election, well I'm in agreement with that bumper sticker that reads "Any Functioning Adult, 2020."
If the "functioning adult" is Bernie - I don't think it will be - I'll have to bite the side of my cheek hard and pull the lever for him.
The nominee will not be Andrew Yang either. This is OK and supporting him in consistent with my personal history over a long lifetime of voting. As far as I can recall, I have never supported a candidate in the primary season who won the nomination, so Senator Warren should be glad to be rid of me, if one believes in Karma. In fact, I have never supported a candidate in the Primary season who came close to winning the nomination, except in 2008, when I supported Ms. Clinton.
Bill Richardson, Fred Harris, so and so and so and so, all more or less forgotten, garnered my early attention and support, usually based on their ideas.
So why Yang?
1) His idea about the place of technology. We may have forgotten this, but the benefits rapid growth in labor productivity in the early and mid 20th century were distributed. Workers experienced a work week that contracted to 40 hours; health benefits, vacation time, and access to good schools and safe homes. Yang is the only candidate who seems to understand this, and his value is raising this point in the campaign; it is in fact, a perspicacious point, and - although it dates from the 20th century - in this time constitutes a real and rare, "new idea." This idea needs attention. It is critical in the coming age of AI and robotics.
2) His ideas about climate change will actually work, inasmuch as he supports nuclear power. Ms Warren's stated ideas, which are anti-nuclear will not work, and are in fact dangerous. I note however, that Obama's 2008 energy ideas, involving coal based Fischer Tropsch chemistry, a key stone of Jimmy Carter's energy program in the 1970's would not have not worked; they would have been a disaster. Fortunately President Obama was very different than Democratic Primary Candidate Barack Obama. He actually hired a first rate, world class scientist as his secretary of energy.
Yang's support of nuclear power is involved with the somewhat fashionable thorium/U-233 fuel cycle, but it's OK. The worst nuclear technology is still superior to the best dangerous fossil fuel technology.
3) Mr. Yang is not in my generation, the Baby Boomer generation, which has been the least great generation since that of the antebellum generation preceding the American Civil War. In some ways that awful freak in the White House is an avatar of my generation. Yes, we had good and great people, and some accomplishments, but our post-World War II consumer mentality has been a disaster for our country and for our planet.
4) Yang has no chance of winning the nomination. I need to be consistent. My support for a candidate will have no bearing on the outcome. I live in New Jersey. Before we have a primary, the candidate will be more or less decided.
That about sums it up in a nutshell.
Have a nice weekend.
Nature Commentary: Climate tipping points -- too risky to bet against?
The commentary I'll discuss in this post comes from the prominent scientific journal Nature: Nature 575, 592-595 (2019)
In the title of this post I have added a question mark that is not included in the commentary, which is not to say that I question the point, but since the commentary is written by European climate scientists and is written about or to inspire government policy, it is increasingly clear, from simple measurements of carbon dioxide concentrations in the planetary atmosphere, that the public does not take the risk even remotely seriously.
Even on the middle class and upper class left, where we nominally accept the science - one cannot "believe" in science, since scientific facts do not depend whether or not the majority of people are intellectually or emotionally equipped to "accept" them - we think that we can continue to live in our sybaritic ecstasy if only we embrace electric cars, and continuously cheer for the vast areas of the planet being destroyed to build industrial parks for wind "farms," while mining, often under appalling conditions using appalling processes, vast amounts of chemical elements for transmission lines, solar cells, and other useless junk misnamed "renewable energy."
So called "renewable energy" did not work, it is not working and it won't work to address climate change.
This is experimentally observed: World Energy Outlook, 2017, 2018, 2019. Data Tables of Primary Energy Sources. If one accepts science rather than "believes" in science, or particularly if one is trained in science, one understands that if one has a theory, and the experimental results conflict with the theory, the theory is wrong, and not the experiment.
The results of the multi-trillion dollar so called "renewable energy" experiment are in: The use of dangerous fossil fuels and the accumulation of dangerous fossil fuel wastes - only one of which is carbon dioxide - is now at the highest rate ever observed in human history, with the 1st derivative of such use and accumulation also being at the highest rate ever observed and the second derivative is uniformly positive.
From what I can tell the commentary is open sourced, and I will only excerpt a few brief passages, before making some remarks on the public perception of the all important topic of risk.
From the first few paragraphs:
Here we summarize evidence on the threat of exceeding tipping points, identify knowledge gaps and suggest how these should be plugged. We explore the effects of such large-scale changes, how quickly they might unfold and whether we still have any control over them.
In our view, the consideration of tipping points helps to define that we are in a climate emergency and strengthens this year’s chorus of calls for urgent climate action — from schoolchildren to scientists, cities and countries.
The Intergovernmental Panel on Climate Change (IPCC) introduced the idea of tipping points two decades ago. At that time, these ‘large-scale discontinuities’ in the climate system were considered likely only if global warming exceeded 5?°C above pre-industrial levels. Information summarized in the two most recent IPCC Special Reports (published in 2018 and in September this year)2,3 suggests that tipping points could be exceeded even between 1 and 2?°C of warming (see ‘Too close for comfort’).
The commentary begins with the word "Politicians." I note that two of the authors come from countries whose governments have endorsed and support the offshore drilling of dangerous fossil fuels, Denmark and Great Britain, and another comes from a country that has absurd and extremely dangerous energy policies, Germany.
A few other excerpts:
I referred to some of this palaeo-evidence elsewhere in this space:
The amplitude and origin of sea-level variability during the Pliocene epoch
Thus, we might already have committed future generations to living with sea-level rises of around 10?m over thousands of years3. But that timescale is still under our control. The rate of melting depends on the magnitude of warming above the tipping point. At 1.5?°C, it could take 10,000 years to unfold3; above 2?°C it could take less than 1,000 years6...
Future generations...as if we gave a shit.
As well as undermining our life-support system, biosphere tipping points can trigger abrupt carbon release back to the atmosphere. This can amplify climate change and reduce remaining emission budgets...
In 30 years of personal research, I have convinced myself that the only viable solution to address climate change is nuclear energy. It is the only technology with a high enough energy to matter density to slow the first derivative, change the sign of the second derivative, and perhaps create a negative first derivative for the presence of at least one dangerous fossil fuel waste, carbon dioxide, although the latter change represents a vast engineering problem that cheap carny barkers like, say, Elon Musk, engaged in marketing ersatz "solutions," are far too ignorant to comprehend.
Smoke another joint Elon...
The world now has well over 17,000 reactor years of commercial nuclear operations.
There have been three major failures, two of which involved the release of volatile radioactive components to the environment. I hear about them all the time, generally from people with a clear and obvious inability to think straight, and they are all more famous than the 7 million people who die each year. They are, chant after me: Three Mile Island, Chernobyl, and Fukushima.
A fourth putative "disaster" is the Hanford nuclear weapons plant in Washington State, to which I am often directed by stupid people to consider - even though I have clearly considered this plant on a far deeper level than most of these dumbbells who raise the point with me - my favorite and most memorable such occasion being an ignoramus who told me that I should be OK with 7 million air pollution deaths each year because a tunnel collapsed on the Hanford site with "radioactive materials" in it.
Thank God DU has an ignore function. The anger such ignorance raises for me is not good for my health.
The causes of all three of the major nuclear reactor failures can be, in a straight forward way, engineered away, and all technology is subject to failure, and any technology involving the use of high energy is subject to failure involving a loss of life. The issue is whether, on balance, a technology saves more lives than it ends.
For many years, I heard that the "solution" to the variability of so called "renewable energy" was transmission lines, made of copper sheathed in polymeric species suspended from steel towers. Now California, which bought heavily into the "renewable energy will save us" theory, which has failed to address climate change, has experienced vast destructive fires from an extensive network of, um, transmission lines.
Does this issue get as much coverage, or any coverage, comparable to Fukushima, the latter being an issue that any man or woman on the street is aware?
Three major failures of nuclear reactors do not impress me. The experimental probability of a major failure is observed to be 3 failures/17,000 reactor years = 0.02%. The experimental probability of a reactor failure resulting in the release of significant quantities of radiation is 2/17,000 = 0.01%. Again, these failures show us a path to engineer away their risks. I have made myself familiar with almost all of them.
Another widely employed engineering product is aircraft. It is well understood by simple appeal to deaths/passenger km (or mile) that flying is far safer than driving a car, or even riding a bicycle where cars exist. Nevertheless, deaths from aircraft accidents greatly exceed deaths from nuclear reactor failures and what we do when such deaths occur - as detailed in the wonderful engineering show on the Smithsonian Channel, "Air Disasters" - is to engineer away the risks. We do not as a culture, declare air travel too dangerous. But the real risk of air travel is contained in the fact that it is powered using dangerous fossil fuels., the waste of which is proving intractable.
The overwhelming share of posts I make on this political website refer to the primary scientific literature, and of these, the overwhelming share is devoted to issues in climate change, either the reality of climate change or to possible engineering processes to address it, or, are directed at debunking, with appeal to scientific research and scientifically collected data, the incredible and deadly popular enthusiasm for proposals that have not worked, are not working, and will not work to address the extreme risk of irreversibly destroying the entire planetary biosphere, or at least rendering it unrecognizable to cognizant species.
Does the general public understand this risk? I think not at all. All day yesterday and most of the night, I walked through one of the world's largest and most prominent cities, lit up with electronic signs selling stuff, some of them as high as 50 meters. No where was there any reference to climate change, although there were many ads encouraging people to buy plastic stuff, metal stuff, or to take aircraft to remote regions of the planet for fun.
Are the risks of climate tipping points to risky to allow to continue? The answer from the world seems to be "stuff it."
I hope you're enjoying the Thanksgiving weekend.
Heavy Lanthanides: An "Imminent Crisis."
The paper I'll discuss in this brief post is this one: Heavy rare earths, permanent magnets, and renewable energies: An imminent crisis. (Karen Smith Stegen, Energy Policy 79 (2015) 1–8)
I came across it going through some old files (accessed in 2018), but had not read it, although the subject of critical elements has been a subject of considerable interest to me, and represents a big part (besides toxicology and wilderness preservation) of why I changed my mind on the issue of whether so called "renewable energy" is sustainable. It is clear enough that despite all of the mindless cheering for it (in which I, to be honest, used to participate) so called "renewable energy" has not addressed climate change, is not addressing climate change and, I contend, will not address climate change. This paper addresses that issue.
From the introduction:
...This article seeks to serve as a wake-up call to renewable energy advocates, whether government officials, policy makers, industry decision-makers or simply concerned citizens. We begin by providing background information on rare earth elements and permanent magnets, clarify several ubiquitous misperceptions about rare earths and outline the risks of heavy reliance on a single supplier. We then review and assess the various methods for addressing shortages and present the main issues associated with developing rare earth supply chains outside of China. The article closes with a discussion of the implications and several policy
recommendations.
The bold is mine, which is to reflect my feeling which would be amusing were it not so dire, that trying to "wake up" advocates of so called "renewable energy" to the fact that so called "renewable energy" is not, in fact, "renewable," is at best a Sisyphean task, but more likely, a Quixotic task.
One thing I have noted about advocates of converting every wilderness area into industrial parks for short lived wind "farms" - the use of the word "farm" is another example of, um, lying - is that they are in general, disinterested in replacing dangerous fossil fuels and are more interested in attacking nuclear energy, even though nuclear energy is the only sustainable form of energy there is and represents the only workable tool for addressing climate change. (As good as nuclear energy is, however, addressing climate change at all is increasingly a long shot.)
The author makes a distinction between the heavy lanthanides and the light lanthanides, which is a very important distinction, and one about which I've spent considerable time thinking, particularly with respect to dysprosium.
Several of the rare earths used in renewable energy technologies and efficient lighting applications are considered critical, that is, at risk for short- and mid-term shortages. The United States (US) Department of Energy (US DOE, 2011) assessed the criticality of various materials to clean energy applications according to a two-part schema: the importance of each individual material and [page 3] the severity of the supply risks. Materials scoring high on both dimensions are considered “critical”, and those at medium or low risk are deemed, respectively, “near critical” or “not critical” (see Table 1). For both the short- (0–5 years) and medium-term (5–15 years) periods, five rare earth elements were placed in the critical category: dysprosium, neodymium, europium, yttrium, and terbium. Most of these are categorized as heavy rare earths: dysprosium, used in neodymium–iron–boron permanent magnets (for example, in wind turbines and electric vehicles); terbium, used primarily in lighting (terbium can also substitute for dysprosium, but is more expensive); and yttrium, used in lighting. Europium, used in lighting, lies between the light and heavy rare earths on the periodic table and is considered a heavy rare earth by some authorities (US DOE (US Department of Energy), 2011, Molycorp, 2012 and Alkane Resources, 2013) and as a light rare earth by others...
Although the chemistry of the lanthanides (aka "rare earths" as in this article) is very similar, which is why all of these elements, plus yttrium and scandium, are generally found together in ores, there is a very subtle but consequential difference in their chemistry which appears when the f shell is half filled, which occurs at europium. Europium itself is sometimes depleted in these ores because it, unlike the other lanthanides, has a very stable +2 oxidation state, which makes its chemistry more like that of barium and strontium than the other lanthanides, effecting the geochemistry of some ores. The elements after europium, except sometimes in some contexts gadolinium, have some differences in their geochemistry which makes changes to their distributions.
The author gives one - among many that are not mentioned in this paper - fairly good xample of the relevance of these elements, including dysprosium, to the utility of so called "renewable energy:
Neodymium–iron–boron magnets are even stronger than samarium cobalt magnets and, because their size is not as restricted, they are more suitable for large applications, such as wind turbines and other electricity generators. These magnets typically contain two to four percent of dysprosium to enhance their temperature resistance. The advantages offered by neodymium–iron–boron permanent magnet to renewable energies are not inconsequential. Depending on the system, permanent magnets can increase efficiency—upwards to 20 percent—which translates into lower costs and shorter payback periods. For example, at least two major benefits can be derived from replacing the mechanical gearboxes in wind turbines with direct-drive permanent magnet generators: first, the overall weight of the turbine is reduced, which thus reduces the costs of other components, such as the concrete and steel required to support heavy gearboxes; second, reducing the number of moving parts allows for greater efficiency and reliability (Hatch, 2014; see also Kleijn, 2012). The advantages of permanent magnet generators are particularly salient for offshore installations, where reliability is paramount due to the high costs of maintaining and repairing turbines. Neodymium–iron–boron magnets are also used in other types of renewable energy technologies—such as underwater ocean and wave power (Dent, 2014). Additional potential applications that could use permanent magnets include small hydro applications, solar updraft towers (Hatch, 2008), geothermal drilling (Hatch, 2009), and heat pumps (rdmag.com, 2013). Several of these renewable energy technologies are in the prototype or testing stages. One factor that could impede their commercialization is the price of permanent magnets. Indeed: were the price lower, many existing renewable energy technologies could be re-designed around them, which could reap the same efficiency, size, reliability and, ultimately, cost benefits as they already produce for new technologies (Hatch, 2014).
Table 1:

The table gives a nice overview of the uses of these elements, omitting a few.
Many of the light lanthanides are fission products, some of which feature radionuclides with acceptably short half-lives that suggest they could be utilized directly after isolation. These are praseodymium, neodymium and lanthanum. The latter two contain radioisotopes that are very long lived and are in fact found in the natural ores as a result, for example Nd-144. Cerium contains the parent isotope of Nd-144, Ce-144, which has a half-life of 284 days, meaning that to utilize cerium in places where the radioactivity would not be desirable - there are many potential applications where the radioactivity would be desirable - would require up to ten years of cooling.
Promethium, element 61, is found only in used nuclear fuel and not in nature (except for very, very minor trace amounts from the spontaneous fission of natural uranium). It is not very long lived in the common isotope, 147, but has been utilized for permanent lighting applications.
Samarium and europium have very high neutron capture cross sections, and, as a result, are somewhat depleted in used nuclear fuels and are, in any case, the reason that nuclear fuel becomes exhausted in the current common nuclear reactors, before all the fissionable material is exhausted. I believe that in "breed and burn" reactors, they might serve (besides as control rods) as long term neutron shields for reactors that run for decades without refueling. Under these conditions, some of these elements would be transmuted into "heavy" lanthanides.
Used nuclear fuel however is not an option for the long term supply of lanthanides, since it has a high energy density.
The amount of plutonium required to meet all of the world's energy demands, shutting all the world's energy mining (including for many centuries, uranium mining or extraction from seawater), all the gas, all the oil and all the coal, is rather small.
Currently the world is consuming about 600 exajoules of energy per year. The amount of plutonium required to meet this demand is relatively trivial, about 7,500 tons per year, as compared with billions of tons of dangerous fossil fuels consumed each year.
These small quantities, and the fact that the lanthanides are only a fraction of the elements that can be obtained as fission products, suggests that the lanthanide problem cannot be solved by isolation from used nuclear fuels, as the yearly production would only represent a small fraction of world demand.
I wish you a happy Thanksgiving holiday.
Anyone having Thanksgiving in a restaurant?
For the first time in about 25 years, my family is.
It's in New York City.
I'm not really crazy about the idea, but some out of town family is flying in to do it, including some people I last saw when they were children and who are now parents themselves.
I found a paper giving the solution for the diffusion equation for a conical boundary. Life...
...is very beautiful, and then you die.
It's just one of those truly wonderful things.
For some reason, I never looked for it, or maybe I did, but didn't know how to look for it.
It all came together in a bout of really, really, really bad insomnia.
Solutions of the diffusion equation in cones and wedges
Bare Metal Critical Masses of Commonly Available Plutonium Isotopes.
The paper I'll discuss in this post is this one: Denaturing of Plutonium by Transmutation of Minor-Actinides for Enhancement of Proliferation Resistance (Saito et al, Journal of Nuclear Science and Technology, Vol 42, Issue 2, 161-168 (2005)).
The ultimate cause of our complete and technical failure to address climate change is selective attention.
And let's be clear, we are failing, we are doing nothing effective to address climate change. Here are the figures immediately available at the website of the Mauna Loa Carbon Dioxide Observatory's website:
Up-to-date weekly average CO2 at Mauna Loa
Week beginning on November 10, 2019: 410.25 ppm
Weekly value from 1 year ago: 408.91 ppm
Weekly value from 10 years ago: 385.76 ppm
Last updated: November 23, 2019
While the figure in comparison to the same week of 2018 seems low, "only" 1.34 ppm over last year, this is only the third such reading in 2019 to below 2.00. The average of such readings for 2019 as compared to 2018 is 2.98 ppm as of this writing, and in the week ending April 8 of this year, the same reading was 4.48 ppm over the same week in 2018. Of the 19 such readings over 4.00 pm, 12 have occurred in the last 5 years, 16 in the last 10 years.
We are failing. Big time.
I blame selective attention for this inasmuch as climate change is a result of our energy production practices and the criteria by which we engage in the comparative analysis is for lack of a better set of terms, completely and totally puerile, silly, and absurd.
As I frequently point out, citing the irrefutable data in this open sourced paper, Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895), nuclear energy saves lives.
This, of course, does not imply that nuclear energy is without risk, that no one has ever been harmed by nuclear energy. This is obviously not true and never will be true. It is, I think, in the presence of a responsible cost analysis that values lost lives from any technology equally, absolutely no feasible way to make nuclear energy safer than it already is, and no rational reason for doing so, since any improvement would be minor and raise costs.
It is not, at least to sane people in my opinion, justifiable to spend tens of billions of dollars to prevent 5 future deaths or 500 future deaths from radiation leaks while we are unwilling to spend the same amount of money to provide improved sanitation to the 2 billion people who lack it, thus allowing 827,000 deaths (the WHO figure) each year from lack of clean water and fecal born diseases, 432,000 of which involve diarrhea, mostly among small children.
I a member of a generation, the so called "Baby Boomers," who proved to be, at the end of their reign, mostly concerned about money and consumption, and little else, except for some tiresome and weak gesturing, half-assed perfunctory sloganeering and cursory lip service.
It is common among members of this generation to declare, without a shred of critical thinking, that "nuclear power is dangerous" because of so called "nuclear waste," about which these same people claim, without having ever opened a science book or a scientific paper in their generally useless lives, that "nobody knows what to do with it."
The reality though is that air pollution, which again, I often point out, kills seven million people per year, and climate change to the extent it is driven by carbon dioxide are both waste problems, fossil fuel and biomass combustion waste.
The difference between so called "nuclear waste," and dangerous fossil fuel waste is essentially this: There is no record of commercial so called "nuclear waste" actually killing people beyond the fantasies of moral morons, and there is an extensive record of dangerous fossil fuel waste killing people. About 800 people will die in the next hour from fossil fuel and biomass combustion waste.
On some level, there is some excuse for Baby Boomers to have focused their fear on radiation, since many of us can remember, albeit when we were children, the Cuban Missile Crisis, when we went to school some mornings in October of 1963 expecting to be vaporized at some point in the day. Nevertheless, our focus on our childhood fears are not really excuses for having lost our ability to think critically.
There are now people who have elevated this childish fear of radiation- every human being is mildly radioactive because every human being would die without the element potassium which does not occur (except in special laboratories) in a non-radioactive form - to engage in hysterical and extremely stupid evocations of say, some crap about the Hanford weapons facility, as a justification for letting seven million people die each year from dangerous fossil fuel and biomass combustion waste. It is not worth even speaking to such moral Lilliputians.
By the way, it is not sane to spend hundreds of billions of dollars to "clean up" Hanford to a standard that no one is ever, for all time, injured by its contents, at the same time that we are unwilling to spend hundreds of billions of dollars to clean up the planetary atmosphere, which is killing people continuously.
This brings me to the issue the authors of the paper cited at the outset of this post, the subject of putative nuclear war. It is a fact that for almost 3/4 of a century, 73 years to be exact, the number of people killed in nuclear wars has been zero. (This is not true of nuclear tests, but it is true of nuclear wars.) It is also a fact that millions of people have died in the last 73 years from petroleum based wars, using petroleum based weapons of mass destruction (and some dangerous natural gas based weapons of mass destruction, since nitrates are synthesized using natural gas). Yet the petroleum industry and the gas industry are not required to prove that their products and materials cannot be diverted to use in weapons of mass destruction, a task which would be futile, since they are continuously observed to do so, but the nuclear industry is so required.
The paper is written about the "Kessler proposal" (and similar proposals) to which I have paid considerable attention since it was published in 2004, which is to limit world plutonium supplies to those containing significant quantities of the 238Pu isotope, a heat generating isotope that has mostly been utilized to power deep space robots investigating the outer (and a few inner) planets in our solar system.
I am a proponent of the uranium/plutonium nuclear fuel cycle to save humanity from itself, but it largely not this cycle discussed in this paper: I propose the fast neutron (breeder) cycle, whereas this paper focuses on the thermal cycle. Inasmuch as the bare sphere metal critical mass of actinide isotopes is very much involved in my thinking, the paper nonetheless caught my eye. This is because I changed my mind a few years ago about whether large nuclear reactors were to be preferred to smaller, easily manufactured reactors: I now think small is better.
The introduction begins with a statement about the reality that selective attention exists:
Later the introduction gets to the Kessler proposal and outlines it:
The authors, citing a previous paper by the lead author, advance this argument further:
Most of the previous researches have been devoted to the effort of increasing 238Pu as way of protection. However, exclusive and separate investigation on the effect of 238Pu on denaturing plutonium would fairly underestimate the combined effect of other even-plutonium isotopes, 240Pu and 242Pu, which have relatively large bare critical mass (BCM) and remarkably large SFNs, since fuel irradiation in LWRs inevitably produces these isotopes.
The present paper therefore focuses on the intrinsic feature of proliferation resistance of plutonium with consideration of the dilution of fissile-plutonium isotope with evenmass- number-plutonium-isotopes. Increasing the fraction of even-mass-number-plutonium-isotopes by plutonium irradiation and MA transmutation in LWRs, the denaturing of reactor- grade-plutonium to unattractive material for fission explosives by utilizing MA transmutation in LWRs is also studied in the present paper...
"SFN" refers to "spontaneous fission neutrons" which are the neutrons released in a subcritical state by the inherent property of both naturally occurring actinides (except thorium) and synthetic actinides to fission spontaneously, without being hit by an external neutron. The presence of to many such neutrons makes nuclear weapon manufacture problematic, and limits their lifetime considerably.
Again, this paper focuses on the thermal cycle, which is not my primary interest, but the tables supporting the discussion are of interest, so I won't discuss much more of the text.
The first table does what the title of this post promises, and gives the bare metal critical masses of plutonium isotopes (presumably spherical geometry, since this is what nuclear weapons utilize).
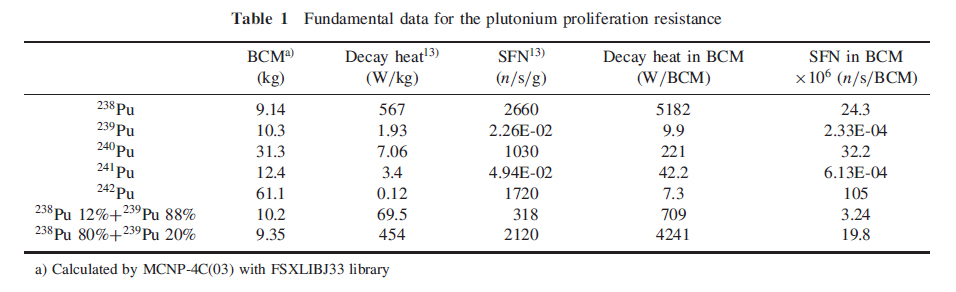
The column BCM stands for "Bare Critical Mass."
The high figure for the decay heat associated with 238Pu, 567 watts per kg, shows why it is useful on space vehicles.
A second table is also of interest since it shows the composition vectors of the main actinide constituents of used nuclear fuel.
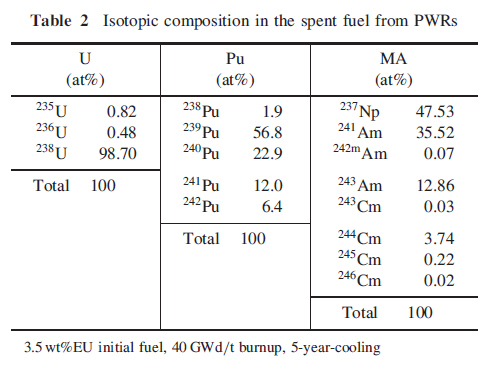
These figures are for "once through" plutonium, that generated utilizing enriched uranium at start up. Of note to me is the relatively low concentration of Pu-242, which is a wonderful isotope since it is fairly but not completely (as it has a critical mass) inert nuclide. Its presence has the effect of increasing the critical mass of any plutonium sample containing it.
In the area of nuclear engineering in which I focus most of my attention, specifically liquid metal fuels, this has an important consequence in terms of a difficult issue, which is heat transfer.
Plutonium is one of three metals exhibiting a very long thermal liquid phase temperature range; the other two are neptunium and gallium. This means it is not easy to get plutonium to boil (although it does so in nuclear weapons explosions). I have in my library a report dating from 1966, when a generation less stupid than mine was working on breeder reactor concepts - indicating that the specific power of a liquid plutonium fueled reactor (the LAMPRE reactor) was 1MW/kg. (cf. Whitman, "Fast Breeder Reactor Development in the United States" in Fast Breeder Reactors, Proceedings of the London Conference on Fast Breeder Reactors, 17-19 May, 1966, P.V. Evans, Ed. page 286.)
Presumably the plutonium utilized in this reactor was or almost was weapons grade plutonium, nearly pure 239Pu. It was, however in the form of an iron plutonium eutectic. The density of this fuel at 760°C, the temperature at which the reactor was said to have operated is approximately 15.53 g/ml. (cf Wittenberg and Ofte, Fluid Flow of Liquid Plutonium Alloys in an Oscillating‐Cup Viscosimeter J. Chem. Phys. 48, 3253 (1968)) The eutectic point in the iron plutonium system exists at about 10% iron, suggesting that at this power density, if we assume that the 1MW/kg figure refers only to the plutonium content, involved 70 ml of fuel putting out 900 kW of energy.
The LAMPRE reactor utilized tantanlum crucibles, and small amounts of plutonium, and thus heat transfer may not have been an issue, if, in fact, this 1MW/kg figure is real, which it may not be.
This suggests a role for 242Pu in reactors of this type, which is to lower the energy density of the fuel.
There is nuclear fuel available with a higher 242Pu content, largely available in France, which is "twice through" MOX. The vectors I've seen for it, show much higher concentrations of 242Pu.
I hope the French protect this fuel. It will be important for future generations to have it.
Have a nice day.
Identity and Toxicity of Off Gases in Thermolysis Lithium Battery Recycling Schemes.
The paper I'll discuss in this post is this one: Toxicity Identification and Evolution Mechanism of Thermolysis-Driven Gas Emissions from Cathodes of Spent Lithium-Ion Batteries (Fang Hu et al, ACS Sustainable Chem. Eng. 2019, 7, 22, 18228-18235).
Over the decades, I've encountered many members of the awful class of human beings to which I belong, "Baby Boomers" - a class that never really escaped from the "Baby" designation - who, when confronted with either an environmental disposal issue or to a limited resource issue, engage in hand-waving coupled with the magic word, "recycle."
I am not immune from this mentality of course; much of what I write on the internet is about recycling, in particular, the recycling of the components of used nuclear fuels, and recovery of the valuable components therein.
Of course, there really isn't much used nuclear fuel in the world - certainly less than would be desirable in a safe and sustainable world - and in pure terms of mass they physical transport of these materials would be, were we sane (and we're not) relatively trivial. In the United States, for example, the world's largest producer still of nuclear energy, after over 60 years of operations, less than 80,000 tons of used nuclear fuel exists. We may compare this with the hundreds of billions of tons of the dangerous fossil fuel waste carbon dioxide the United States has released in the last 60 years, and the hundreds of millions of tons of waste plastic, which despite all the rhetoric about "recycling" is clogging the guts of fish and other marine, lacustrine, and fluvial organisms and will soon, in terms of mass, exceed the mass of all the living marine, lacustrine and fluvial higher organisms.
The "recycling" of plastics did not work, is not working, but I think could work, albeit only with entirely new thinking of a type that isn't fashionable.
Nevertheless, despite our obvious failure to be responsible, we Boomers like to declare ourselves "green." We Baby Boomers like to tell lies, to ourselves and to others, witness that awful orange Baby Boomer creature sliming the White House with his insipid and criminal presence, who is, in some ways, the avatar of our generation. He is often portrayed and mocked using a giant orange plastic balloon, inflated with some of the last helium on Earth, wearing a diaper, which is amusing and perhaps proper in terms of marketing, but unfortunate in ways we often don't consider.
Even wit has environmental consequences.
If I differ from other Baby Boomers in magical thinking about recycling, it is only because I work to make myself aware of the practical aspects or recycling, many of which are far less "green" than we like to imagine, because first of all "recycling" requires significant amounts of energy and although a few of us know how to make clean energy, in general, as a practical matter, we don't do so.
One of the most intractable forms of waste is electronic waste. It turns out that when we engage in magical "recycling" of electronic waste, the actual performance of the task is consigned to poor people in areas about which we neither know or care to know, precisely because the risk and toxicology of the recycling technologies are dangerous to health of those performing the task and destructive to the environment, and as such, is not permissible in countries with sensible environmental regulations, regulations that diaper Don and his denizens are working to eliminate.
One of the big Boomer lies we tell ourselves and others is that we can make so called "renewable energy" viable if only we had enough batteries to store the energy generated by this pixilated scheme. So called "renewable energy" has not been viable, is not viable and won't be viable, and we will never have enough batteries to make it so. Nevertheless, we are accumulating batteries at an accelerating pace, and it is that which this paper discusses.
For the record, until recently, China was the most prolific electronic recycling nation in the world; the concentration of polybrominated diphenyl ether and other types of flame retardants, heavy metals like lead and cadmium, and similar substances in the plasma and other tissues of large numbers of Chinese children reflects the results of that "green" industry. As China is becoming rich and powerful, it has banned the import of electronic waste from other countries, and presumably we in the Western world will need to find other poor countries about which we couldn't care less to bear the environmental and health costs of our "green" ways.
Nevertheless, China, now becoming a rich country, has its own endogenous electronic waste problem, and it also has lots of practicing scientists to evaluate the consequences of the practice of recycling electronic waste.
From the introductory text of the paper:
Nowadays, many pyrometallurgical, hydrometallurgical, and bioleaching approaches have been proposed in order to recover valuable metals from the EoL LIBs.(8?11) In a typical pyrometallurgical process, valuable metals are enriched into alloy phases before being subsequently recovered via hydrometallurgical refining processes. Nonetheless, in recent years, state-of-the-art hydrometallurgical processes have increasingly become the preferred industrial method for recovery of a majority of valuable metals within the EoL LIBs cathodes.(12) Pretreatment, either via mechanical or chemical means, is required in order to make the different waste battery streams ready for the leaching operations that follow. Of these, thermal-based pretreatment processes have become widely adopted and have been the subject of numerous research in recent years.(13,14) For instance, Sun and Qiu(15) and Yang et al.(16) have suggested that heat treatment of the cathodes can lead to the active powder being completely separated from Al foils. Hanisch et al.(17) have proposed that the thermal decomposition of organic compounds like PVDF binder can reduce the cohesion of the coated particles and weaken the adhesion between the particles and Al foils. In our previous investigation, it was found that thermal pretreatment not only promoted the removal of the organic compounds but also benefited the subsequent leaching of valuable metals.(18) Nevertheless, the risks associated with the toxic off-gas emissions generated during thermolysis remain poorly investigated, and consequently, more detailed information on on the nature of such gas emissions is essential for the development of a more sustainable EoL battery recycling process.
Currently, a number of studies have been undertaken to illustrate the thermal runaway event of LIBs under abusive conditions, whether mechanical, electrical, or thermal.(19,20) During the thermal runaway, battery components, especially the electrolyte, are usually subjected to uncontrolled exothermic reaction chains, which can cause severe safety issues.(21,22) Consequently, the gas emission during thermal runaway of LIBs and its associated mechanisms are relatively well documented, whereas in contrast the gas emissions from a conventional EoL LIBs recycling process has received limited research. Recently, Diaz et al.(23) measured the generated off-gases during thermal and mechanical pretreatment of spent LIBs and found that the thermal pretreatment had superiority to avoid disordered dispersal of the organic substances into the subsequent processing steps. On the other hand, their results also suggested that the off-gases—composed of HF, COF2, acrolein, CO, formaldehyde, HCl, and electrolyte—were of acute toxicity...
COF2 is fluorophosgene. In my career I worked with chlorophosogene, most commonly called simply "phosgene." Phosgene is a war gas that was used to kill tens of thousands of soldiers in the First World War; flourophosgene is more reactive, and more toxic than the phosgene I know so well. HF is hydrofluoric acid. I worked with that too. The safety videos for working with HF include examples of where this acid burned right through to the bone of people who unknowingly had drops of HF solutions on their skin. It is advisable to wear a lot of protective clothing when working with it.
Apparently though, we need more batteries to be "green."
HF, by the way, is a useful reagent for recycling nuclear fuels, but the mass of nuclear fuels is much, much, much, much lower than the mass of batteries when one compares their existing inventory.
The authors used some wonderful analytical chemistry instruments to study the thermal recycling of lithium ion battery cathodes:
Beautiful stuff, although the reality is that all of these wonderful instruments will all some day be electronic waste in their own right, although I have personally been in laboratories featuring workable instruments that are many decades old, although usually these instruments are of a very special type.
Here's a graphic of the schematic of one analytical system:

The caption:
The batteries used in this study were real electronic waste, obtained from a real plant:
Here is a graphic of the DSC/TGA curves. The inflections and peaks represent points at which decomposition chemistry is observed:

The caption:
The mass spectra gives information about the chemical composition of these gases:

The caption:

The caption:
Ion current loosely corresponds to the concentration of the gases, but is a function of the ionization efficiency of the gases and thus the response factor, and is therefore not entirely quantitative without an internal standard.
The following graphic gives some idea of the elemental composition of the solid residues:

The caption:
There is a considerable amount of fluoride ion present, which is a good thing, since the volatile fluoride species are surely nasty. These fluoride ions are almost certainly present as the salts of the metals. Note that this data is in weight percent, and thus reflects the atomic masses of the elements in question. The conflict metal cobalt has an atomic weight of 58.933 grams per mole; lithium an atomic weight of 6.93 grams per mole. Thus on a molar basis, the lithium portion of the graphic, in green should be 8.5 times larger when normalized to the conflict metal cobalt, in purple.
Not all of the fluorine remains behind in the solid however; which is unsurprising, since the hexafluorophosphate ion that serves an electrolyte in lithium batteries (as the lithium salt) is made by reacting toxic pentafluorophosphorous gas with hydrogen fluoride (hydrofluoric acid) gas.
The following sets of equations show the reversal of this inorganic synthetic reaction, present as off gases in the thermolysis of the cathodes:


Nasty.
Flow rates of the off gases:

The caption:
A nice cartoon of the entire study's results:

The caption:
An excerpt from the paper's conclusion:
...…detailed characterization of the gaseous thermolysis products in conjunction with the quantitative changes of the solid products taking place as temperature was increased allowed the evolution mechanisms of the gas emissions from the cathodes to be determined. Also, the evolution mechanisms of the gas emissions driven by thermolysis under various temperatures were established. The solvent EC/EMC was found to volatilize and decompose into gaseous hydrocarbons, CO2, and H2O at around 177 °C. The electrolyte was the main source for the release of fluoridecontaining gases which are mainly composed of POF3 and HF over the same temperature range. The PVDF binder decomposed as H2O, CO2, and fluoride-containing gases with maximum emission peaks at 522 °C, whereas the generation of CO2 above 600 °C was attributed to the oxidation of acetylene black. The analytical data gained about toxic gas species and the evolution mechanism of gas emissions during the thermolysis process provide fundamental knowledge and useful guidance for the continuing investigation of sustainable battery recycling strategies.
The most recent Nobel Prize in Chemistry was awarded for the development of lithium ion batteries. It was well deserved. These batteries have proved to be useful tools in many areas.
Nevertheless it is a lie we tell ourselves to say that batteries will save the world.
They will not.
As this research shows, the idea that this particular type of battery is infinitely sustainable is dubious.
History will not forgive us, nor should it.
I hope you will have a pleasant weekend and enjoy the upcoming Thanksgiving holiday.
Critical Masses of the Three Accessible Americium Isotopes.
Despite the fact that the overwhelming number of war deaths in the last century have resulted from the diversion of petroleum products to make weapons of mass destruction, and zero deaths have occurred from the diversion of commercial nuclear fuel to make weapons of mass destruction, a great deal of attention has been paid to the concept of "critical mass" of actinides, as if it were simple to make nuclear weapons.
Anti-nuke rhetoric, which has made it technically unfeasible to address the far more serious and far more likely disaster scenario of mass destruction by climate change, albeit less instantaneous, as opposed to wholesale nuclear war, often includes silly and frankly absurd calculations about "how many" nuclear weapons can be built from used nuclear fuel.
The reality is that no one can make a nuclear weapon in one's kitchen, and without sophisticated and expensive equipment, anyone handling actinides other than uranium, a naturally occurring product, would face extreme danger to their person without taking extensive precautions.
This is why terrorists like the right wing zealot Timothy McVeigh, and the Saudi Arabian 9/11 terrorists utilized petroleum based weapons of mass destruction, diesel fuel and (dangerous natural gas generated) ammonium nitrate in McVeigh's case, and jet fuel in the Saudi terrorist's case.
As I pointed out elsewhere, it will never be possible to make nuclear war impossible, since uranium exists, and it will never be possible to consume all of the uranium on Earth: On Plutonium, Nuclear War, and Nuclear Peace
The number of extant critical masses that people who are, frankly, complete idiots, utilize in their appeals to fear and ignorance with respect to nuclear wars to advocate against "going nuclear" to address climate change - even though it is the only feasible means of doing so - has an alternate meaning however. The number of critical masses obtainable also represents the number of small nuclear reactors that can be built in a "breed and burn" sense which would render all of the world's depleted uranium and all of the world's waste thorium into valuable fuels with the potential to shut all energy related mining facilities indefinitely.
I favor the uranium/plutonium nuclear fuel cycle on the grounds that it is infinitely sustainable. I don't have anything against the thorium/U-233 cycle, but I think that because of geochemical quirks, it is possible to imagine the depletion of recoverable thorium resources because of thorium's low solubility in seawater when compared to uranium.
For many years, a few decades actually, I considered that there was not enough plutonium to immediately displace all the world's dangerous coal, dangerous petroleum, and dangerous natural gas. When I became aware of the "breed and burn" concept however, I realized that "enough" is simply a function of the number of available critical masses, meaning that the elimination of the use of dangerous fossil fuels is possible almost immediately, in the absence of stupidity, although the absence of stupidity may itself be inaccessible.
There is "enough," particularly in the desirable case of nuclear weapons disarmament.
Because used nuclear fuels have foolishly not been reprocessed over the more than half a century of accumulation in an atmosphere of fear and ignorance, much of the extremely valuable plutonium-241 formed in them, has been allowed to decay to Americium-241. Americium-241 is often thought to be a "difficult" nuclide, because it decays, producing a significant heat load in the process to neptunium-237, which in turn, is theoretically mobile in putative waste dumps, although the idea of having waste dumps for valuable nuclear fuel should be regarded as absurd, since all of the components of used nuclear fuel represent valuable resources.
Except for uranium-238 and thorium-232, both of which occur naturally in vast quantities, and some very rare isotopes of actinium, present in trace amounts in uranium ores, all of the actinides subject to isolation in macroscopic amounts have a critical mass in a fast (unmoderated) neutron spectrum.
As a nuclear fuel, Americium lacks some of the attractive features (to me anyway) present in plutonium and neptunium as nuclear fuels. Specifically the melting point of Americium is higher than either of these two actinide elements. (A plutonium/neptunium eutectic has the lowest melting point among the actinide elements subject to isolation.)
Nevertheless, as a source of denaturing isotopes of plutonium, plutonium-238 and plutonium-240 and plutonium-242, the use of americium fuels has much to recommend it.
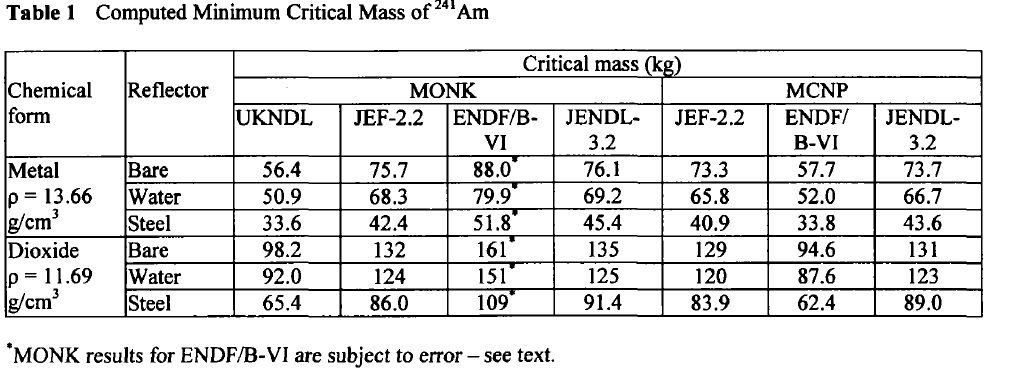
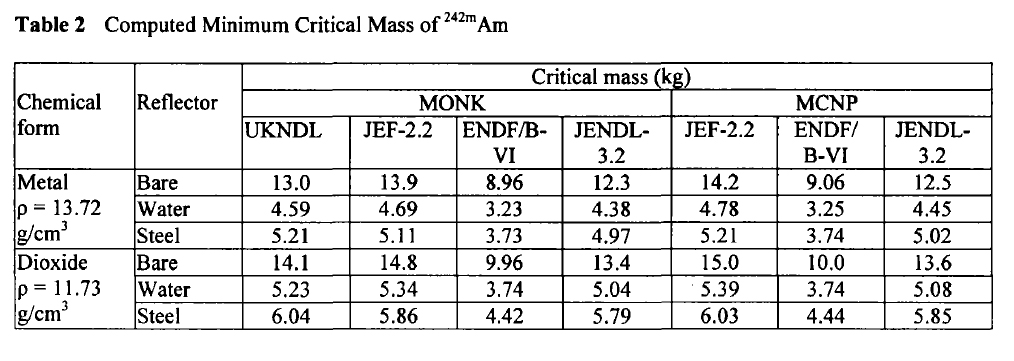
In 2003, at a conference on nuclear fuels, British authors, Henmanth Dias, Nigel Tancock, and Angela Clayton, offered a paper that recalculated the critical masses of Americium isotopes.
Three such isotopes will appear in Americium isolated from any source, Am-241, Am-242m, and Am-243. It is exceedingly difficult to separate the isotopes of americium, since it does not form volatile compounds. The shortest lived isotope just listed is Am-242m, which has a half-life, off the top of my head, of around 152 years. It is the least available of all Americium isotopes, since the majority of the 242 isotope formed in nuclear reactors is Am-242, the nuclear isomer of Am-242m, which has a half-life, again off the top of my head, of 16 hours. The 242 isotopes, both of them, are the most fissionable of all Americium isotopes.
Here are the calculated critical masses of Americium isotopes using various nuclear codes:
Am-241:
Am-242m:
Am-243:
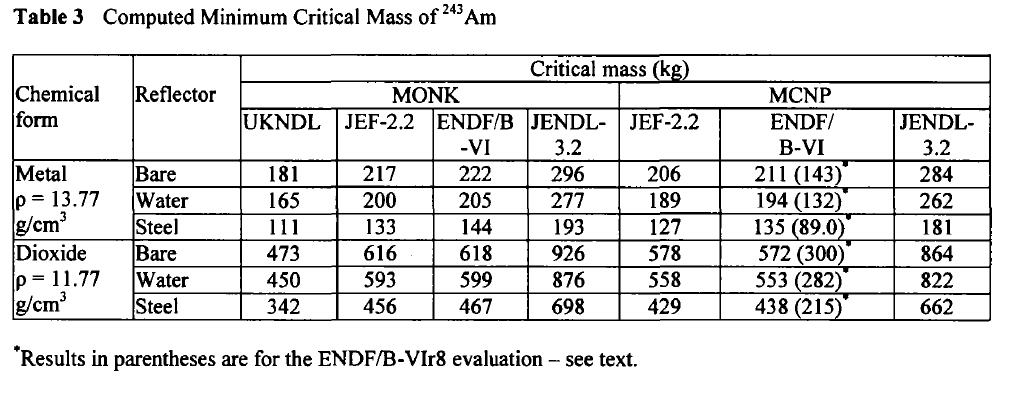
These tables are from the Proceedings of the JAERI conference 2003, pages 618-623.
I hope your weekend is wonderful; and that your plans for the upcoming Thanksgiving holiday are proceeding satisfactorily.
Distribution & Type of Marine Debris Polymers on Hawaiian Island Beaches, Sea Surface, and Seafloor.
The paper I'll discuss in this post is this one: Marine Debris Polymers on Main Hawaiian Island Beaches, Sea Surface, and Seafloor (Jennifer M. Lynch et al. Environ. Sci. Technol. 2019, 53, 21, 12218-12226).
As bird populations fall, accelerated by the wondrous goal of converting all of our continental shelves into industrial parks for wind farms, as well as because of the topic of this post, plastic, a part of the phosphorous cycle will be disrupted, specifically the sea to land portion. Many of the world's mined sources of phosphorous are actually bird droppings on Islands. For a short while, the island nation of Nauru in the Pacific Ocean had the world's highest per capita wealth in the world because it exported bird shit, phosphorous, deposited by sea birds over centuries. (The Nauruan Government "invested" all of this wealth in stocks and bonds which collapsed, and now the nation is one of the world's poorest, the bird shit is depleted, and the Island makes its living by imprisoning refugees deported from Australia.) The importance of birds to the phosphorus cycle is described in the interesting book Why Birds Matter, CAGAN H. SEKERCIOGLU, DANIEL G. WENNY, AND CHRISTOPHER J. WHELAN, Eds., University of Chicago Press, 2016, pp 274-275, 279-282.
I mention this, because I often think about the recovery of important elements and compounds from seawater by raising it to supercritical temperatures. This would serve to recover both phosphorous and carbon dioxide in cases where the seawater is dead from deoxygenation owing to agricultural run-off, as in the Mississippi River Delta, the ecosystem of which has been destroyed by runoff to make "renewable" corn ethanol. The eutrophication process which killed it, involves the explosive growth of micro-organisms which sink to the bottom of the sea as they die after getting killed off by the thickness of the mats they form which restricts sun light, are rotted by oxygen depleting bacteria, killing everything else, fish, crustaceans, and other species.
When I muse on this subject of supercritical water oxidation (SCWO) to recover phosphorous and carbon dioxide, I often reflect that a side product of the process would be to destroy microplastics, which are contaminating the ocean in ever larger amounts, and as another side product would be fresh water, since at supercritical temperatures and pressures, seawater separates into two separate supercritical phases, one containing salts, and one free of salts.
Future generations may need to do these sorts of things, because we have screwed them.
(I may discuss a few interesting papers I came across on polymer reprocessing engineering I just came across that were published in the last few days; not processes I necessarily endorse, but interesting engineering nonetheless, in future posts here.)
One thing I had not considered in my musings is the density of plastics, which is a topic covered in the paper under discussion.
From the paper's introduction:
Since Hawaii accumulates debris from a variety of sources, understanding the chemical composition of plastic marine debris is necessary.(15) Seven standardized resin codes are assigned to the most commonly produced polymers: (16) polyethylene terephthalate (PET, #1), high-density polyethylene (HDPE, #2), polyvinyl chloride (PVC, #3), low-density polyethylene [LDPE, #4, which includes linear low-density polyethylene (LLDPE)], polypropylene (PP, #5), polystyrene (PS, #6), and other polymers (#7). Some consumer goods are stamped with their resin code, but weathered fragments are often missing these stamps, requiring chemical analyses for identification.
Polymer identification of plastic marine debris is crucial for understanding sources, fate, transport, and effects in the environment. Because different polymers have various chemical structures, their physical, chemical, and biological interactions within the environment will differ. Sorption rates and concentrations of organic and heavy metal pollutants vary among polymers, making certain polymers a greater threat of contaminant exposure to organisms.(17) Chemical reactions during environmental degradation processes can lead to various polymeric degradation products that have not been widely studied.(18?23) The release of additives, fillers, and greenhouse gases(21,24) are highly variable among polymer type and in some cases even toxic.(25,26) Polymer identification tools also provide indicators of the extent of the debris weathering, a sign of aging or possibly a time estimate since littering.(20,27) Each polymer has a different chemical density, which is hypothesized to be a major (but not the only) influence in vertical stratification and fate of plastic debris in the ocean (Table 1).(28,29) For instance, polymers less dense than seawater (e.g., PE and PP) float and are commonly found at the sea surface,(30?34) while denser polymers predominantly sink to the seafloor.(29,35,36) In addition, polymer identification can confirm that debris samples are in fact plastic and other material is not visually mistaken as plastic.(37) These reasons, plus the need to understand which polymers may affect different marine habitats, provided justification for the present study.
The authors collected plastic samples from seawater, from the beaches, and the benthic zones of the Hawaiian islands.
They were collected by divers, by collecting plastics in trawlers, and by picking them up the beaches. The types of plastics were determined simply, by FTIR, using a Perkin Elmer library. (An alternative, and possibly superior approach to polymer identification is differential scanning calorimetry, DSC, but FTIR is pretty good.
Here's a description of the handling of the samples and the samples themselves:
...um...delicious...
A little more on polymer ID:
Anyway, here is the table, from the paper, detailing the density of various plastics.

Here is a map of the sampling site beaches:

The caption:
(The authors studied the effect of land development on beach plastic accumulation (see the excerpt below).
Debris amounts are higher in the MHI than many other places. Ribic et al.10 reported that Oahu has higher debris loads than the US Pacific coast. MHI beaches sampled in the current study were more plastic polluted than South Korean beaches (means = 13.2 items/m2 and 1.5 g/m2 of 0.5?2.5 cm each)53 even though they sampled additional particles in smaller size classes (<1 cm), which inflates their abundances compared to the current study. The current results are also 2 orders of magnitude greater than the North Atlantic Azores (0.62 pieces/m2 of >2 cm) of a similar size range.54 It is challenging to compare the present data with published debris abundances on beaches because of the differences in particle sizes targeted. This emphasizes the need to report multiple measurements (piece counts, size distributions, and mass) to understand the type of debris in a region...
The abundance of debris:

The caption:
Where the plastic ends up by form:

The caption:
The degree of weathering (probably somewhat subjective).

The caption:
Figure 4. Weathering rank of MHI plastic marine debris across compartments, percentages of pieces. Debris on the seafloor and leeward beaches (A) are less weathered than windward beaches and the sea surface (B) (MRPP, p < 0.0001). Values are mean ± one SD.
Composition by area of collection:

The caption:
Polymer composition as a function of the type of debris.

The caption:
The relationship between land use and polymer concentration is an interesting discussion:
These correlations could be confounded by beach cleanups, but we believe that this possible confounder is a minor variable. Cleanup effort is undoubtedly higher on tourist beaches, such as Waikiki, but large-scale cleanup events are scheduled frequently for the less developed beaches. The exact timing of cleanup effort before our sampling was often unknown. Kahuku on windward Oahu has less land development, is located within the James Campbell National Wildlife Refuge, and received the largest debris amounts of all sampling sites.14 Portions of Kahuku are cleaned up approximately weekly to monthly. It was obvious that a recent cleanup had occurred at one of our three Kahuku transects. Still, Kahuku had the highest debris abundance, suggesting that recent cleanup had little impact on our overall findings.
Percent land development and weathering intensity showed a strong negative correlation (Figure S10B, Pearson R2 = 0.600, p = 0.0051). Waikiki, the most developed, had the least weathered debris, suggesting that the small abundance of debris on this beach is from local sources with minimal exposure to environmental conditions. The least developed beaches (Kamilo, Lanai, and Molokai) had the most weathered debris. Weathering intensity for pieces exposed to sunlight could reflect environmental exposure time. The more weathered pieces on the sea surface and windward beaches were in the environment longer, arriving to Hawaii via wind and ocean currents from distant sources, compared to more recently littered debris on leeward beaches.
Types of debris were correlated with land development (Figure S10C). More fragments were found on less developed beaches (Pearson R2 = 0.362, p = 0.050), while more sheets were found on more developed beaches (Pearson R2 = 0.443, p = 0.025). Fragments are formed from mechanical and chemical weathering after extended environmental exposure. As such, the less developed windward beaches received debris dominated by fragments that were presumably washed ashore from older litter of distant sources...
There is quite a bit in the full paper, and, in any case, it certainly is sobering to contemplate this mess we're leaving for future generations.
While supercritical water oxidation may serve to reduce floating polymers it's not clear how to address sunken or buried polymers, and in any case, the industrial infrastructure to do this would need to be massive, and utilize sustainable energy, which does not include the solar and wind industry.
The paper's conclusion:
Scary, but interesting.
Have a nice day tomorrow.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509