NNadir
NNadir's JournalConsidering an Alternative Hybrid Allam Heat Engine Cycle for the Removal of CO2 from the Air.
In this post, I will consider two papers from the primary scientific literature. Both are about a form of energy I oppose but nonetheless has proved to be the fastest growing source of energy on the planet in this century: Coal. I trust my discussion of these papers will not in any way distract from my often stated position that coal is unacceptably dangerous and should be phased out, along with the other two dangerous fossil fuels, dangerous petroleum and dangerous natural gas, beginning immediately, on an emergency basis.
The papers are:
Parametric study of a direct-fired supercritical carbon dioxide power cycle coupled to coal gasification process (Zhang, Wang, Chi, Xiao, Energy Conversion and Management 156 (2018) 733–745).
Atomistic Simulation of Coal Char Oxy-Fuel Combustion: Quantifying the Influences of CO2 to Char Reactivity (Yongbo Du,†,‡ Chang’an Wang,†,‡ Haihui Xin,‡,§ Defu Che,† and Jonathan P. Mathews, Energy Fuels 2019, 33, 10, 10228-10236).
Coal, of course, is nothing more than sequestered carbon, carbon that was sequestered over hundreds of millions of years from biomass. In a few generations, roughly in two centuries, humanity has more or less de-sequestered the bulk of it, producing the dangerous fossil fuel waste carbon dioxide which is dumped into the atmosphere without charge, is rapidly destroying the entire planetary atmosphere.
The largest single contributor to the 7 million air pollution deaths that take place while dumb guys carry on about how dangerous nuclear energy is, almost certainly derive from coal. Since coal is nothing more than sequestered and carbonized biomass, it is unsurprising that the second largest contributor to these air pollution deaths is likely to be fresh biomass, probably followed by deaths from air pollution related to dangerous petroleum.
Thus the relationship to historical fossil biomass, coal, and modern fresh biomass is close, with coal being somewhat more dangerous than "renewable biomass" since aqueous solutions of certain toxic metals, for example lead, mercury, uranium and cadmium have leached through coal resulting in their extraction from water and their concentration in coal formations. This is why the other toxic coal waste, coal ash, is such a toxicological nightmare, because the ash contains these metals, concentrated over thousands upon thousands of millennia.
Although in general I oppose so called "renewable energy" because it is not environmentally sustainable, and because it is rather dirty and destructive to both wildlife and to pristine wilderness rendered into industrial parks, it is nonetheless true that one form of so called "renewable energy" represents an opportunity to re-sequester the carbon released by the combustion of dangerous coal. This is of course, biomass. I personally believe that it is feasible to engineer away some of the more odious and baleful effects of the use of biomass to produce energy, hence my interest in the Allam cycle.
The earliest commercial nuclear reactor in the Western world, unlike the bulk of nuclear reactors operating today (with some exceptions), did not use a water/steam system as the working fluid to drive turbines in order to generate electricity. This reactor was the Calder Hall nuclear reactor in Great Britain, which began construction in 1954 and came on line in 1956, and operated until 2003. The Calder Hall Reactor used carbon dioxide as a working fluid. As Great Britain was at the time a nation which generated the bulk of its electricity from coal, the Calder Hall reactor ran for almost half a century saving human lives that otherwise would have been lost to air pollution.
The Calder Hall reactor was a very innovative device in its time, built largely on 1940's and early 1950's technology, but one of the first devices not only to be powered by nuclear fission, but also to use as a working fluid carbon dioxide, thus offering certain thermodynamic advantages to be discussed below in an excerpt of the paper cited above by Chinese authors.
The Allam Cycle is a thermodynamic cycle, a modification of the Brayton cycle by which jet engines and a number of dangerous natural gas power plants operate. It developed by an Englishman, Rodney Allam, and is being piloted and developed by a company called "8 Rivers Capital" in North Carolina. It also uses carbon dioxide as a working fluid, but with a twist, the working fluid is also the combustion gas, with the combustion taking place not in air, but rather in pure oxygen, that is an oxyfuel setting.
I believe I have discussed the environmental advantages of oxyfuel combustion here and elsewhere on the internet. By substituting pure oxygen for air the combustion chamber, one can achieve very high combustion temperatures, high temperatures being an condition which always raises the Carnot efficiency of power plants and also allows for certain types of industrial chemical processing, in an extreme case, for example, the production of concrete precursors. (The manufacture of concrete is a huge contributor to climate change.) The other advantage is the near elimination of nitrogen oxides as a component the combustion of dangerous fossil fuels (and for that matter, biomass) waste, said nitrogen oxides being currently a huge environmental problem. The largest advantage is however, is that the combustion gas is almost pure carbon dioxide, greatly simplifying the recovery of the gas. Disadvantages of oxyfuel combustion are largely related to corrosion effects in the materials of the combustion chamber. The temperatures are high enough to volatilize salts, among other things, and any steam resulting from the combustion will likely be (depending on pressure) in a supercritical state, where water it quite acidic, pH ? 3, and thus corrosive.
On some level, the Allam Cycle is obvious - I've certainly had similar ideas over the years - and it's quite possible that materials science issues will impact the viability. Nevertheless it seems quite possible that variants might be of interest for issues in climate change.
As being developed by 8 Rivers Capital the Allam Cycle is clearly focused on continuing the use of dangerous fossil fuels, with the lipstick on the pig being the idea of sequestering carbon dioxide in giant carbon dioxide dumps that are frequently discussed as a potential "solution" although in reality they have not been built, are not being built and hopefully never will be built on any appreciable scale. This said, carbon dioxide is being utilized to produce more dangerous fossil fuels, for example in "Enhanced Oil Recovery" operations and similar fracking type dangerous natural gas recovery operations.
The construction and photographs of the construction of pilot Allam cycle plants can be found in a paper co-authored by Sir Rodney himself. It is here: Demonstration of the Allam Cycle: An Update on the Development Status of a High Efficiency Supercritical Carbon Dioxide Power Process Employing Full Carbon Capture☆ (Allam et al, Energy Procedia 114 ( 2017 ) 5948 – 5966) The pictures in this paper, which I will not reproduce here, show plants being constructed in partnership with Toshiba. The marketing goals that 8 Rivers Capital are exploiting to raise money for this enterprise are also listed, they are these:
2. The global market for new and replacement fossil fuel plants through 2040 [24]
3. The needs for CO2 for enhanced oil recovery [25].
4. The needs for CO2 for enhanced coal bed methane recovery [26].
The realization of any of these marketing goals with the possible exception of part of goal 2, would represent a continuation of the ongoing disaster for humanity, although the money raising aspect should not obscure the potential real value for doing what the conference at which it was presented stated was supposed to endorse. The conference was called "13th International Conference on Greenhouse Gas Control Technologies, GHGT-13, 14-18 November 2016, Lausanne, Switzerland"
Building carbon dioxide dumps in lieu of the one now being used, the planetary atmosphere, is not controlling carbon dioxide by the way. It is very much the same thing as what we are doing now, dumping the costs and responsibility for cleaning up after our lifestyle on future generations. In any case it won't happen. We are dumping more than 35 billion tons of carbon dioxide each year. Thus the idea that we can contain this gas forever, this possibility often being raised by ignoramuses who contend that we cannot contain 75 thousand tons of largely solid (and generally valuable) used nuclear fuel, assembled after half a century of operations without costing a single human life, is so absurd as to be considered insane, but somehow isn't so considered.
In any case, the Energy and Management paper gives a nice overview of the reasons for the thermodynamic superiority of carbon dioxide in comparison to water, after a burst of truth about the fact that, despite what you may have heard, coal is not dead, far from it. The data from the 2018 IEA World Energy Outlook report shows that despite much ballyhoo offered by provincials in the United States, where the coal industry is declining and being replaced by "only" half as bad dangerous natural gas, coal has been the fastest growing source of primary energy on this planet in the 21st century. (The 2019 World Energy Outlook will come out on November 13; I personally don't expect it will produce a significantly different result - coal experienced a very modest decline between 2016 and 2017, but the decline was completely and totally trivial, and easily erased by increases in the use of dangerous petroleum and dangerous natural gas.)
The nice overview from the introduction, despite the grammatical artifacts of its translation from Chinese into English, to the Energy Conversion and Management paper:
The direct-fired supercritical carbon dioxide (sCO2) power cycle is one such promising candidate. By combustion of the fuel gas with stoichiometric oxygen and recycling CO2 as the combustor temperature moderator, the working fluid of the power cycle is highly enriched in CO2, with its molar concentration well above 90% [5]. The sCO2 based power cycles are well known for their high efficiency potentials [6]. The high efficiency comes from the superior physical property of CO2—the moderate critical point at 30.98 °C and 73.8 bar [7]. The much lower critical point of CO2, compared with that of water, facilitates the utilization of the unique thermodynamic advantages brought by the supercritical fluid. On the one hand, as a consequence of the low critical temperature, the compression of the sCO2 power cycle could occur near the critical point, an area where the physical property experiences abrupt variation, especially the density [8]. The working fluid in this area behaves more like liquid rather than gas. The compression work can thus be much reduced and the efficiency enhanced. On the other hand, owing to the low critical temperature again, the isothermal evaporation or condensation process of the working fluid is avoided in the cycle.
The Allam cycle is not particularly different than a “normal” Brayton cycle plant, in which an exhaust gas, derived from the combustion of fluidic dangerous fossil fuel directly drives a turbine except that in the Allam cycle the carbon dioxide component is compressed rather than exhausted to the air, and then recycled back into the fuel.
The recycling of carbon dioxide back into the fuel has the property of slightly cooling the fuel while simultaneously “dry reforming” the dangerous natural gas. This is because at high temperatures, carbon dioxide becomes and oxidant for methane in an endothermic reaction in which three carbon dioxide molecules react with a molecule of methane to give two molecules of water and four carbon monoxide molecules. If the conditions are correct from a mass balance and energy perspective, two of the carbon monoxide molecules can be reoxidized to carbon dioxide while the water is reduced to hydrogen gas; this is the water-gas-shift reaction, the water gas shift reaction being the reaction by which almost all of the world’s hydrogen is produced using dangerous natural gas.
The resultant hydrogen/carbon monoxide mixture is known as “syngas.” Basically any large scale organic commodity in the world obtained using dangerous petroleum can more or less be synthesized using syngas. A common use, run at various times in the 20th century on an industrial scale (and once proposed by Jimmy Carter for US government support when he was President to break the stranglehold of OPEC) is for the Fischer-Tropsch reaction the “FT reaction.” This reaction can make synthetic gasoline and/or synthetic diesel fuel and synthetic jet fuel, fuels which burn slightly cleaner than petroleum-based diesel. Of course, saying “slightly cleaner” about these dangerous fossil fuels or a putative substitute is like saying that it is better to have lung cancer than pancreatic cancer, since lung cancer patients live slightly longer than pancreatic cancer patients, but no matter. The main Fisher-Tropsch application today is to make synthetic motor oil which is designed to run in cars for very long periods is generally made using Fischer-Tropsch type chemistry. The synthetic motor oil lasts longer than motor oil refined from dangerous petroleum because its chemical constituents can be more tightly controlled.
The carbon source need not be dangerous natural gas. The original Haber process for the preparation of hydrogen to make ammonia – a reaction on which until this day the world food supply depends – and the Fischer-Tropsch fuels that drove the Nazi war machine in the latter parts of World War II, used coal as the carbon source.
This brings me back to the first paper, from which the introduction was excerpted, in which syn gas and electricity is produced using dangerous coal.
Here, for convenience is a table listing many, but not all, the abbreviations utilized in the text.

Here is some text about the CO2 capture option being explored for this dangerous coal plant, with reference to earlier considerations:
An interesting aspect of this paper caught my eye, which is concerned with the topic of materials science implications of a very high temperature gas expanding against a turbine.
My personal view is that clean power plants should be designed to last for a period approaching a century, and the extent to which they do so is very much dependent on individual components. Great advances have been made in recent years in understanding that it is possible, in a what is called a "breed and burn" setting to build nuclear reactors that will not require fueling for many decades of operations, that can run at full power for periods of at least half a century, and it does seem to me that longer periods are conceivable. Since the number of nuclear plants required would be reduced by raising efficiency, and the use of nuclear power to remove carbon dioxide from the air and to reduce it, to reverse climate change, would necessarily require very high temperatures - cerium based carbon dioxide splitting requires for part of the cycle temperatures of approximately 1400°C - the issue of the temperature of gases and their effect on the integrity of turbines is always on my mind. This is why this paper, which is about a form of energy I hate, is of so much interest to me.
A diversion on turbines: The Brayton cycle is in wide use and they have very much depended on the temperature resistance of turbines, both in every jet engine on the planet and in "combined cycle" dangerous natural gas plants. Almost all of these turbines are manufactured using nickel based superalloys that, while being designed to function at high temperatures, routinely encounter gases that are at temperatures that are significantly higher than the melting point of these alloys. This problem is overcome by the use of thermal barrier coatings. An excellent paper discussing this subject was written by the interdisciplinary scientist (and Dean of the Engineering Department) Dr. Emily Carter of Princeton University on the occasion of her induction into the National Academy of Scientists: Atomic-scale insight and design principles for turbine engine thermal barrier coatings from theory (Kristen A. Marino1, Berit Hinnemann2, and Emily A. Carter3, PNAS April 5, 2011 108 (14) 5480-5487) (A major theoretical paper where all three authors are women, each of them with intellectual power that people having pig brains - I'm talking about you Brett Kavanaugh and Donald Trump - are too ignorant and stupid even to imagine! Cool!)
(Regrettably, the dependence of hafnium as a the solution suggested by Dr. Carter to the binding issue of the thermal barrier coating to superalloys is probably not sustainable simply because hafnium may be regarded as a "critical element" subject to depletion as we steal the future from future generations.)
Anyway.
The maximum allowable turbine blade temperature assumed in the paper now under discussion is 860°C, lower than the melting points of many available superalloys, but no matter, this is not the real point of the paper in any case. (The performance of superalloys is not entirely connected with melting, the solvus point, in which the components of the solid solutions, that the alloys represent, separate is also important. Temperatures approaching 1400°C but still below it are observed among a few commercial superalloys - at least as of 2010 - for example CMSX-10, which reportedly has a solvus temperature of 1345°C. cf. Table 4.3, page 45, Geddes, Leon, Huang, Superalloys: Alloying and Performance.)
Again, this is a paper about coal, and the application to which I would like to see this technology applied (and not necessarily involved with combustion so much as reforming using nuclear heat) is biomass. In this paper, it actually turns out that there is a way - important for materials science considerations - in which the coal under discussion is actually cleaner that biomass. Here is the elemental composition of the Chinese coal under consideration for the purposes of this evaluation, Datong bituminous coal:

Here, for comparison, is the elemental composition of Maize Straw Ash (also Chinese) from a paper I discussed in a recent post in this space:

cf: Influence of Sewage Sludge on Ash Fusion during Combustion of Maize Straw (Liu et al, Energy Fuels 2019, 33, 10, 10237-10246)
The big difference is the presence of chlorine, which in the scanning electron microscopic/energy dispersive analysis of the ash contained at least one particle that was clearly almost pure potassium chloride. A big problem with corrosion induced by the combustion (or reforming) of biomass is connected with volatile chloride salts, both sodium and potassium chloride. This problem is not observed in the Datong bituminous coal, which is not to say that the coal is acceptable; it isn't.
Anyway, from the paper, here is the process flow engineering diagram of the full Allam cycle plant under consideration:

Some interesting features of this plant include the cyclone, to separate the dangerous coal ash from the gasification of dangerous coal, the fact that the syngas is burned, and not separated for use to make materials or portable fuels, and the ASU, which is an air separation unit, with the air separation requiring additional energy.
There is another way to obtain pure oxygen other than air separation, which is the thermochemical splitting of either water or carbon dioxide or both. (The thermochemical splitting of carbon dioxide into CO and O2 gas is indirectly capable of splitting water into hydrogen and oxygen via the water-gas reaction, by which almost all the hydrogen on Earth is currently made, using dangerous fossil fuels as the source, although clearly high temperature nuclear reactors can do the same thing in an almost infinitely cleaner way.)
Some commentary on the turbine limitations (in this study):

where K1 is a parameter that reflects the turbine geometry and operation condition, which needs calibration according to the working fluid and the turbine operation condition; TIi is the inlet temperature of each expander i; TCi is the temperature of coolant stream i; WEXP?i is the power generated by expander i. The pressure drop is calculated according to the following equation:

where pOi?pIi+1 is the pressure drop caused by mixing the main stream with the coolant; K2 and K3 are similar with K1 that need calibration;mCi is the mass flow rate of the coolant; VHi is the volume flow rate of the working fluid at the inlet of VALVE i. The values of K1, K2 and K3 are directly taken from literature [21]. The allowable turbine blade temperature TW is assumed as 860 °C. The number of the cooled expansion steps N should be a reasonably large number, as a requirement of the continuous expansion model. The recommended value of N is 15 by literature [21]. However, the influence of N on the estimated coolant mass flow rate is not provided. In this study, the influence of N is investigated using data (see Table 2) of the working fluid and coolant for the CO2 turbine presented in literature [21]. The result is shown in Fig. 3.
Figure 2:

Figure 3:

The turbine cooling is provided apparently by expansion of the gases, but there are certainly other options, including a heat exchange network, a topic widely discussed in the literature in many papers that I come across. Heat recuperation is a feature discussed in this paper. Here's a figure of about heat recuperation:

There is considerable discussion in the text of the effect of pressure drops (part of the adiabatic expansion) on the overall thermodynamic efficiency of this system as well as with other components of the system, for instance pumps. Engineering graphics produced below willshow the results of these calculations, the effect of pressure and inlet temperature on efficiency, but perhaps will be of interest only to people with either an engineering or scientific background.
We say on the left that we care about climate change, but given the importance of the problem, we are entirely too glib about it. As a scientist, I am frankly appalled by our unwillingness to seriously consider the problem beyond cruising to silly websites about solar and wind power. Solar and wind power are nothing more than lipstick on the dangerous fossil fuel pig. They have done nothing to address climate change, are doing nothing to address climate change, and will never do anything to address climate change.
Anyone who really cares about the tragedy of climate change and the appalling consequences of what it will do to all who come after us, should be interested in science and engineering, or if they can't be, should at least step out of the way of those who are so interested.
To further make this point I'd like to turn to what should be a disturbing table from this paper saying something about mass flows. This is it:

This power plant is a 1400 MW thermal power plant. At the stated efficiency that the paper estimates in the conclusion, 38.21%, this suggests that the plant would produce about 535 MW of electricity.
According to the International Energy Agency's 2019 Electricity Information Statistics the world produced in 2017, 25606.25 TWh of electricity. This works out to 92.2 exajoules of pure electricity. Electricity demand and production fluctuates widely and thus it is somewhat disingenuous to speak in terms of "Watts" although this terminology is widely used - in a completely dishonest fashion - by advocates of so called "renewable energy," the lipstick on the dangerous fossil fuel pig. Nevertheless, for arguments sake I will do just this, speak in terms of average continuous power, as if I were not discussing an inherently variable system 25606.25 TWh, again 92.2 exajoules, breaks down to an average continuous power demand of 2.92 TW. This means to produce this energy using Allam cycle coal plants modeled in this paper, 5,460 plants would need to operate.
The table above indicates that the coal required to run this plant would be 64.93 kg per second. For 5,460 plants, this would amount to 357.2 tons per second or 11.3 billion tons per year of coal. Since the atomic weight of carbon is 12 and the molecular weight of carbon dioxide is 44, and the carbon content of the Datong coal in this example is 56.75% carbon, the "captured" carbon dioxide for which something must be done permanently forever, would be 23.5 billion tons per year, just for electricity.
It is useful to compare the plutonium requirements to do exactly the same thing at exactly the same efficiency, although personally I have convinced myself that nuclear plants can be built that have much higher efficiency. A kg of plutonium contains about 80.3 trillion joules of neutrino free energy. A nuclear plant producing 1400W of thermal energy would thus fission 17.4 micrograms of plutonium per second or in the "percent talk" so favored in the abuse of language so widely utilized by advocates of so called "renewable energy" 0.00002665% as much mass as the coal plant. 5,460 nuclear plants operating at 38.21% efficiency and 1400 MW thermal energy would produce 1.5 kg of fission products per day per plant, which works out to about 3,000 tons per year.
Which is easier to contain or deal with 23.5 billion tons of carbon dioxide gas, or 3,000 tons of largely solid fission products, only a portion of which would actually be radioactive, and many of which would actually have high value?
How is it that we are so abysmally stupid as to not grasp these simple facts?
Above I promised to discuss a second paper, this one, Atomistic Simulation of Coal Char Oxy-Fuel Combustion: Quantifying the Influences of CO2 to Char Reactivity (Yongbo Du,†,‡ Chang’an Wang,†,‡ Haihui Xin,‡,§ Defu Che,† and Jonathan P. Mathews, Energy Fuels 2019, 33, 10, 10228-10236), which is also relevant to the case, since it concerns the behavior of char. Char is available from dangerous coal (and for that matter from dangerous petroleum) of course, but it is also available from heated biomass. In the latter case, this biomass char in an Allam cycle could be utilized to capture carbon dioxide from the air, which is what this post's title suggested.
Before I was banned at Daily Kos for telling the truth, which is that opposing nuclear energy is simply murder, since this truth flies in the face of our dogma on the left, I used to include mildly amusing polls with all of my posts, one choice always being a variant on the statement "NNadir is a liar and..." with the and being a negation of whatever subject my post explored and my assertions connected with it.
(They're cute over there at Kos when they pretend to actually care about science. The science forum over here is far more interesting than anything written there now.)
Today, the "NNadir is a liar" statement will be the statement that I will discuss the paper just cited about char, coal char. I'm not going to do so now.
Perhaps I will discuss it in the future, but I've run out of time, and have already wasted too much time trying to make a point about which perhaps no one really cares. Nevertheless, all this stuff about Donald Trump is trivial inasmuch as it is ephemeral. In less than 20 years, Trump will be almost certainly dead and more useful than he is alive, and will be a footnote to history, a sad footnote, an appalling footnote, but still a footnote.
Climate change will still be with us in 20 years, quite possibly its effects lasting forever, long after Don Jr, Ivanka, Jared and the rest of those terrible people have died, hopefully in prison.
It's well to consider this.
It's not a totally wasted exercise, for me to write this post, however, because every time I write one these posts I learn quite a bit. The Allam cycle is a topic about which I hope to think in the future. My son is in Italy though, picking up some kind of academic award from the Italian government, and the logistics of getting him from school and putting him on the plane has worn me out, and I'm running out the ability to think clearly.
So are we all, all running out of the ability to think clearly.
At least as a result of this exercise, I'll be able to chat up the Allam cycle with my son, since the future is his and since he's smarter than I am, and however many ideas with which I can leave him to explore, will help him to use his talents to do right by his generation, since my generation has done so much wrong to his.
Some engineering graphics from the paper I did discuss in this post:
The efficiency implications of temperature and pressure for various scenarios:


The pumps also exhibit effects on efficiency:


The air separation to produce oxygen also produces an energetic penalty - this can be overcome in a high temperature nuclear thermochemical water or carbon dioxide scheme and actually raise the efficiency of the overall system.

Some aspects of heat networks and heat recovery:



Some tables from the paper:





I hope you're having a very pleasant Sunday afternoon.
My semi-rural township was pure Republican when I moved in. Yesterday the last Republican...
... town council member was defeated. (He'd been on the town council for more than 20 years, and made it a point that he was also the last farmer on the town council.)
The Republicans were so moderate - even endorsing the "solar will save us" meme to prevent a gas line coming through even though this is not a workable scheme (since solar entrenches gas use) - that my son thought of voting for one. (The Democrats here are not as strong on opposing development in this town as we would like and are allowing the creep of suburbia into places it shouldn't go.)
I told him I once thought of voting for a Republican when I was around his age (Jacob Javits) but didn't do so because saying you are a Republican says, more than ever, what your ethical and moral views are, which is to say, non-existent.
He agreed, and has not yet voted Republican in his life.
Great day! Great day!
Everything must change.
LC-HRMS for Qualitative and Quantitative Analyses of Oligonucleotides
A bit of an ad, but I nice quick overview of a cutting edge development in therapeutics:
https://sciex.com/sciex-online-summit/pharma-and-biopharma-videos
I'm referring to the lecture by Dr. Lin-Zhi Chen which can be reached at the link above.
Polymers with controlled assembly and rigidity made with click-functional peptide bundles
The paper I'll discuss in this post is this one: Polymers with controlled assembly and rigidity made with click-functional peptide bundles, (Pochan et al, Nature 574, 658–662 (2019)).
I had a friend and colleague once who left his job because his company was telling him to make peptides on an industrial scale (ton quantities). He told me, offending me slightly, that he wanted to do "something other than dehydration reactions" that is remove water to make chemical bonds.
Those of us who are environmentalists complain, quite justifiably I think, about polymers, because single use plastics (and to a lesser extent multiple use plastics) are fouling the seas, land, and living systems at an increasing rate. Nevertheless in a very real sense, you are a polymer, or better put, a collection of polymers, since almost all of the molecules of which you are made are polymers.
There was a point in my career that I was a peptide chemist, and trust me, the chemistry of peptides (and their synthesis) is considerably more complex than simply removing water, with all due respect my friend's outstanding knowledge of our science. I will tell you that I spent a year of my life dealing with the huge difference between the behavior of aspartic acid and glutamic acid, two natural amino acids that have exactly the same functional groups and differ by glutamic acid being one methylene group longer than aspartic acid.
Anyway this paper caught my eye, both as a one time peptide chemist and as a person very interested in the sequestration of carbon in useful and environmentally more benign polymers.
From the abstract of the paper:
"Click Chemistry" is chemistry, generally organic chemistry, that involves chemical reactions that take place very fast and in quantitative or nearly quantitative yields under easily accessible conditions. "Click" reactions represent only a small subset of known chemical reactions, but they are very important.
From the papers introduction:
A graphic with some images of these polymers along with a cartoon describing an example of the "click chemistry" utilized in assembling these polymers:
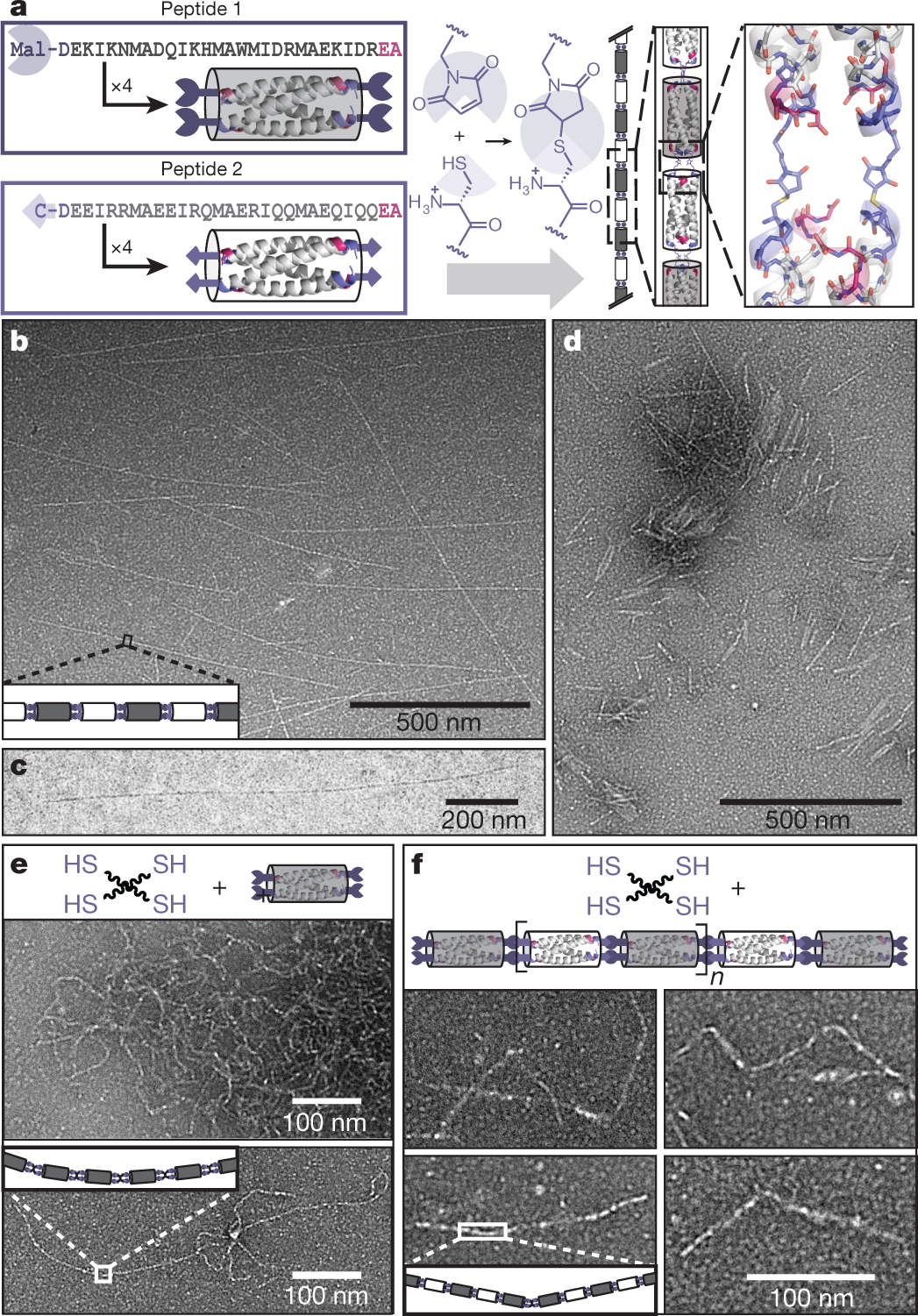
The caption:
In this case the "click chemistry" involves the side chain of a very important amino acid, cysteine, which features a sulfhydryl side chain. (This amino acid is often involved in complexation of metals by metalloproteins, and is a frequently a critical feature of their catalytic sites. The affinity of mercury and cadmium for these thiols in lieu of the zinc with which they are supposed to function is a key factor in the toxicology of these two metals.)
The behavior of some of these polymers as liquid crystals:
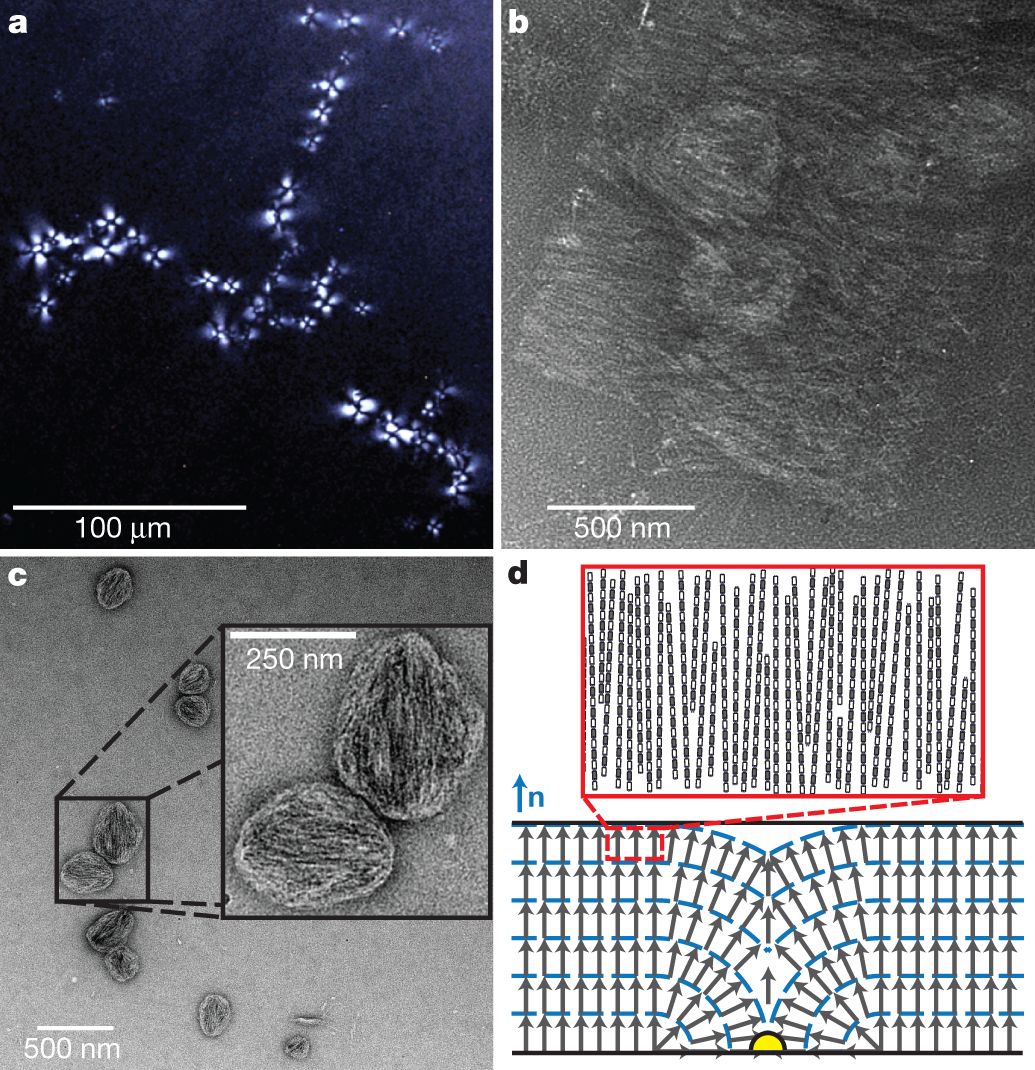
The caption:
Some interesting reversible behavior of some of these polymers:
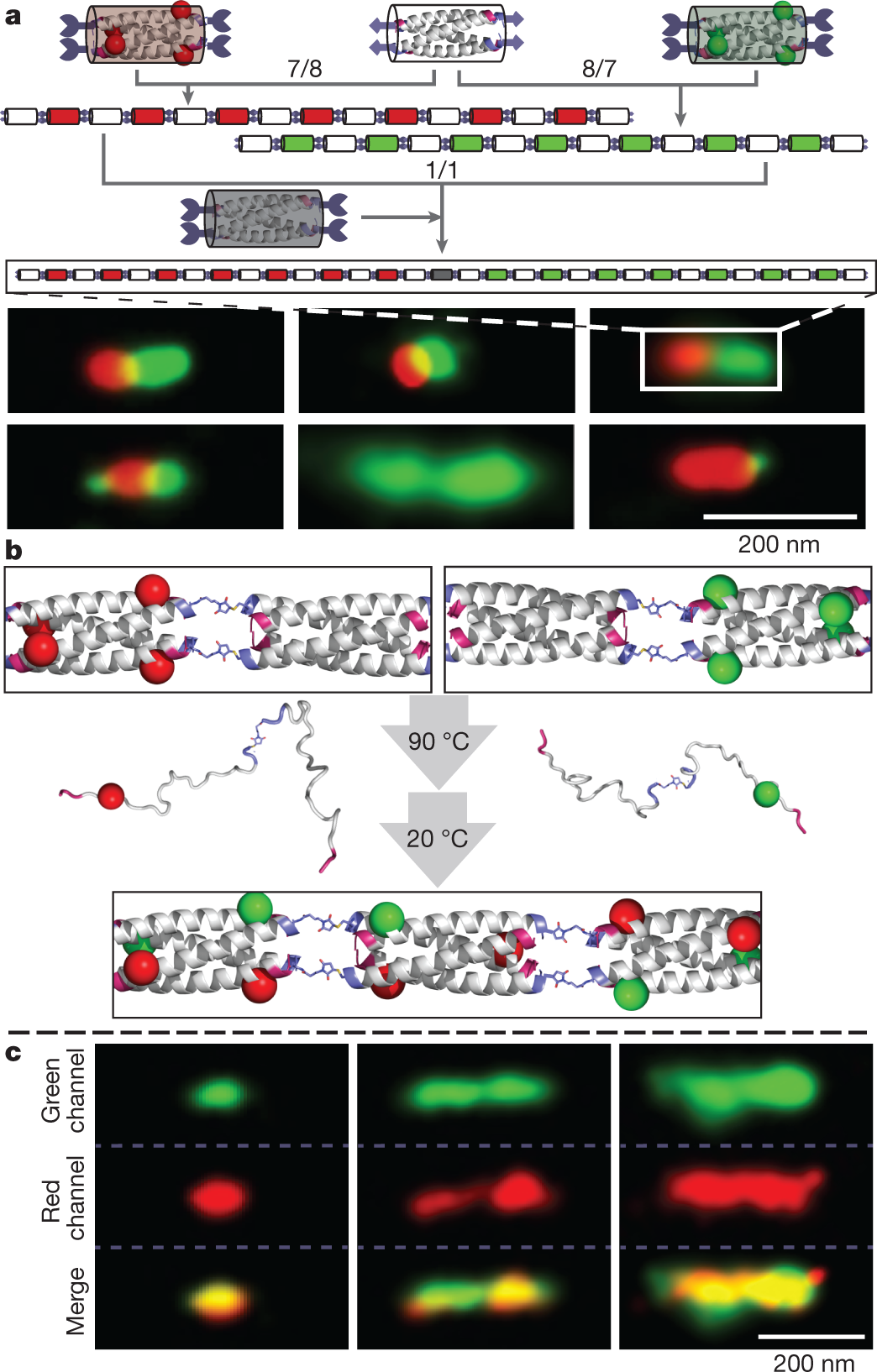
The caption:
Some other interesting properties suggesting hybrid material options:
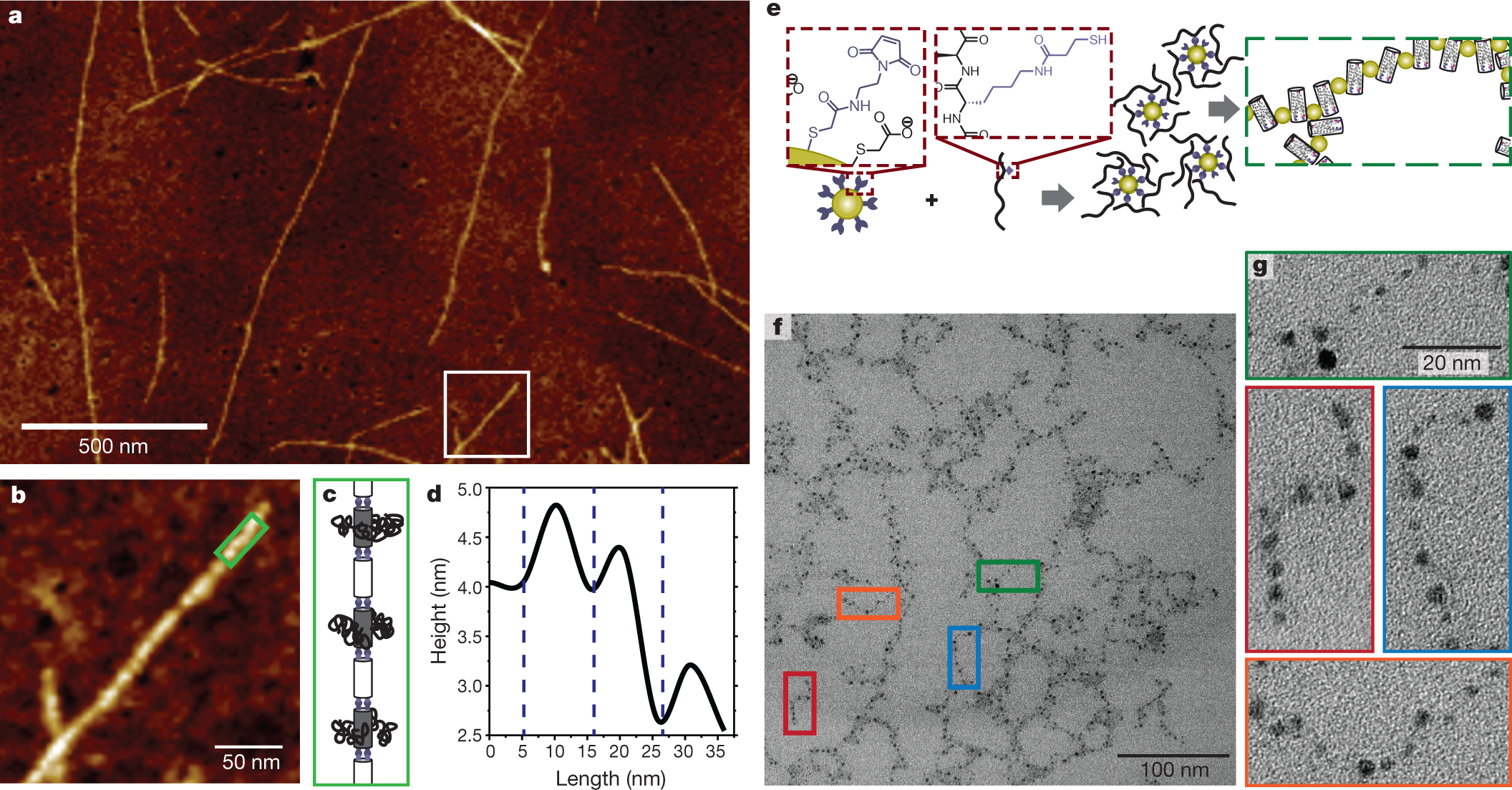
The caption:
Here the co-ordinated nanoparticles are gold, but basically pretty much all of the metallic portions of the periodic table might prove accessible to such controlled polymers, offering the possibility of separations with very high distribution coefficients, useful for recovering dilute materials and also for remediating polluted sites.
Have a nice day tomorrow.
Faradaic electro-swing reactive adsorption for CO2 capture.
The paper I'll discuss in this post is this one: Faradaic electro-swing reactive adsorption for CO2 capture (Sahag Voskian and T. Alan Hatton Energy Environ. Sci., 2019, Advance Article Accessed 10/30/19).
I came across reference to this paper, out of MIT, in the scientific popular press, actually in several places, and since the quality of journalism describing science is often quite bad, decided to access the original paper.
The paper is, happily open sourced, and anyone can read it. I'll excerpt in offer the graphics in any case.
I've spent a lot of time reading about separations of carbon dioxide from various matrices and I will say that this one is somewhat unique, an electrochemical approach. Since the separation of carbon dioxide, a low energy gas, from dilute matrices requires overcoming entropy, this process is not energy neutral by any means; it costs energy, but it may be more efficient. It does not seem operative at air concentrations of CO2, requiring concentrations of 0.6% as compared to 0.041% in air (as of this writing).
The system uses a rather well known organic redox system, dibenzoquinones/dibenzoyhydroquinone in a very creative way
From the introductory text:
In addition to the capture of CO2 from direct combustion processes, there is a need to remove CO2 from enclosed spaces for ventilation purposes in buildings and car cabins, or for cabin environmental control systems on board spacecraft and submarines, where the maximum allowed CO2 concentration in habitable spaces is 5000 ppm (or 0.5%).10 The first of such systems was developed by Winnick et al., for the electrochemical capture of CO2 in spacecraft cabins using molten carbonates.11 However, the low concentration of CO2 in such applications poses a challenge, mainly due to the low driving forces for mass transfer and the large quantities of other species present in air in addition to CO2.12 Thus, carbon capture is a multi-scale problem, where the CO2-rich streams to be treated vary greatly in volume, concentration and composition, and different criteria need to be fulfilled to ensure optimal processing depending on whether sources are industrial or small-scale (e.g., power plants or oil and gas heaters), concentrated or dilute (exhausts from combustion or air in confined spaces), and clean or contaminated with other pollutants.
Many of the CO2-capture chemical processes that involve a capture agent such as amines or solid sorbents require temperature and/or pressure swings to release the captured CO2 and regenerate the agents for further capture. These swings result in inefficiencies due to energy wasted in heating solvents and sorbents, pressurizing feed gas, or drawing a vacuum for desorption. Electrochemical systems can minimize such parasitic energy losses as they can be operated at near isothermal conditions, with significantly higher efficiencies than their thermal-swing (TSA) and pressure-swing (PSA) adsorption counterparts.13 One mode of electrochemical capture of CO2 is through the use of a redox-active carrier.
Electrochemically mediated selective transport of chemical species was first reported by Ward et al.,14 where a redox-active carrier (ferrous ion) was used to transport nitric oxide across a membrane. Since then, a number of systems have been developed for transporting chemical species by redox-active carriers that are activated at one electrode, to bind with the target species, and deactivated at the opposite electrode, to release the target and regenerate the carrier.15,16 Systems that have been proposed for the concentration of CO2 through this approach have been based on a number of different carrier molecules, such as quinones,17–20 4,4?-bipyridine,21 and thiolates.22,23 Quinones are of particular interest to this work for their superior electrochemical performance, serving as redox-active carriers for CO2 in electrochemically mediated separation processes. DuBois et al. demonstrated this possibility, and studied the thermodynamics of an electrochemical CO2 pumping system that utilizes quinones.18 More work followed, where Scovazzo et al. demonstrated the electrochemical separation of CO2 from <1% concentration gas mixtures using 2,6-di-tert-butyl-1,4-benzoquinone as a carrier in ionic liquid (IL) and organic solvent electrolytes media,19 while Gurkan et al. screened a number of ILs to serve as suitable electrolytes for quinone carriers in an electrochemically mediated selective transport system for CO2.20 All of these systems, however, require the transport of the electrolyte and the dissolved carrier molecules between two electrodes in an electrochemical cell for capture and release of CO2. This limits their implementation in a number of applications where the requirement for flow systems and pumping, and the large footprint, are problematic.
The quinones are oxidized and reduced by a porous matrix carbon nanotube (CNT) supported ferrocene polymer.
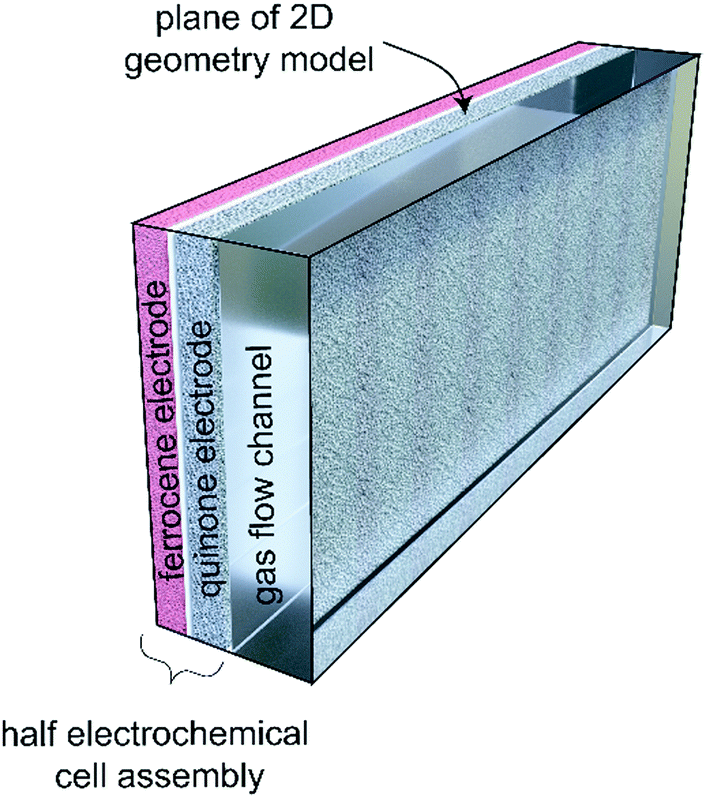
This nice graphic cartoon shows the systems operation.
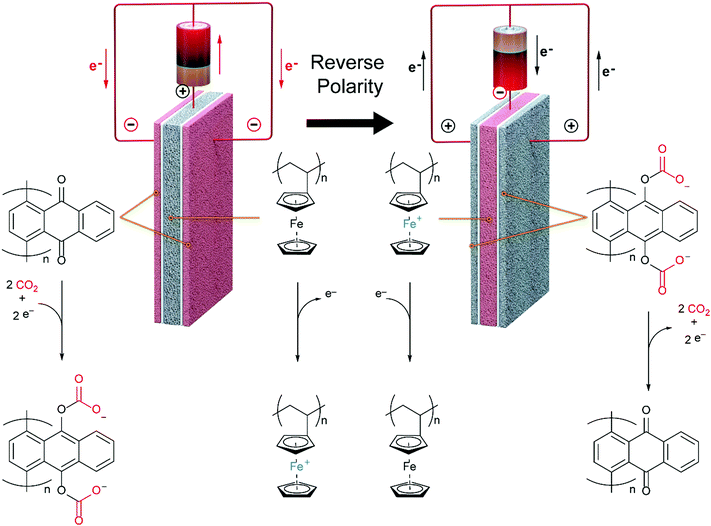
The caption:
This system is designed to treat flue gases, but may be adapted to other types of systems. Recently I've been thinking quite a bit about carbon dioxide as a working fluid for Brayton type devices, and in particular have been focusing attention on a cycle with which I was not familiar until recently, the Allam cycle.
It is a closed cycle, where the combustion gas is also the working fluid.
The Allam cycle is designed primarily for use with dangerous natural gas, but I would imagine that it could also be adapted to other systems, notably those derived from biomass.
During the Allam cycle, portions of the carbon dioxide working fluid are removed from the system, commonly described as being for the purpose of "storage," but coupled with nuclear primary energy, could be utilized for the purpose of making materials, for example carbon nanotubes impregnated with, um, ferrocene polymers, and millions of other similar products.
Anyway, from the paper, some SEM images of the system:
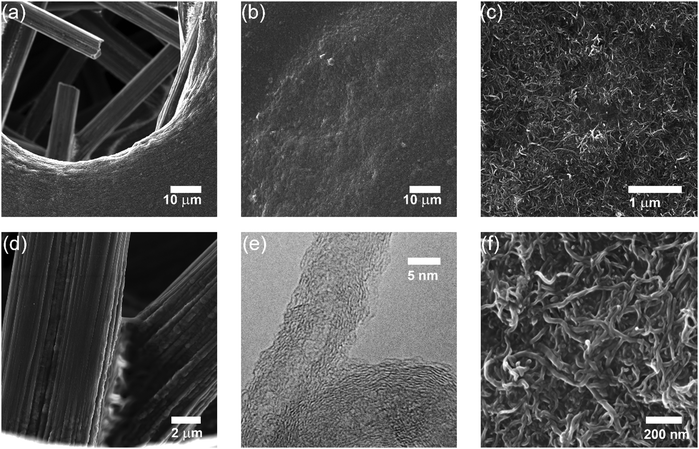
The "? ?" here is reference to the interaction between the aromatic rings of the benzoquinones and those of the carbon nanotubes.
Cyclic voltamograms of the reduction system:
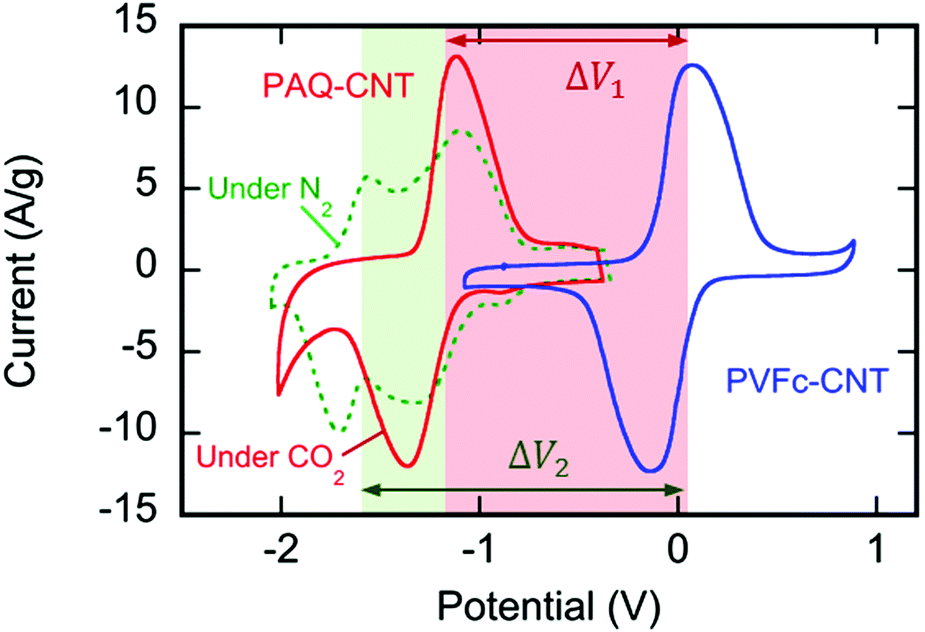
(It is worth noting that increasingly more electrochemical reduction systems for carbon dioxide are known.)
More SEM images:
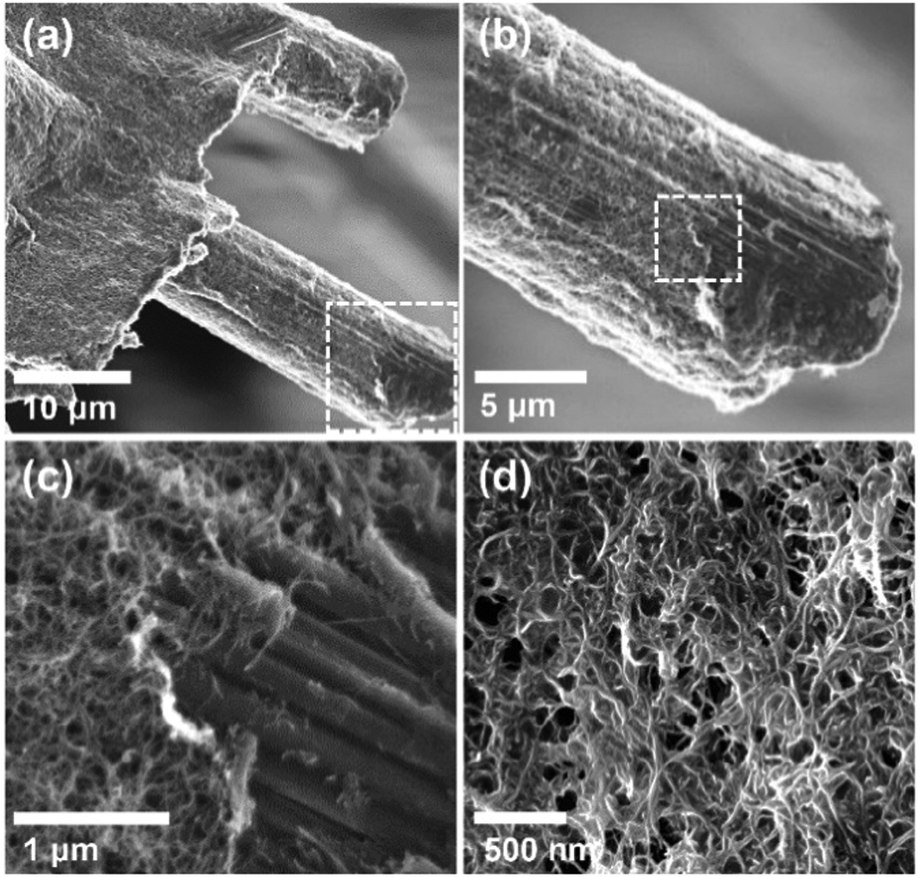
Nice photographs of the experimental apparatus:
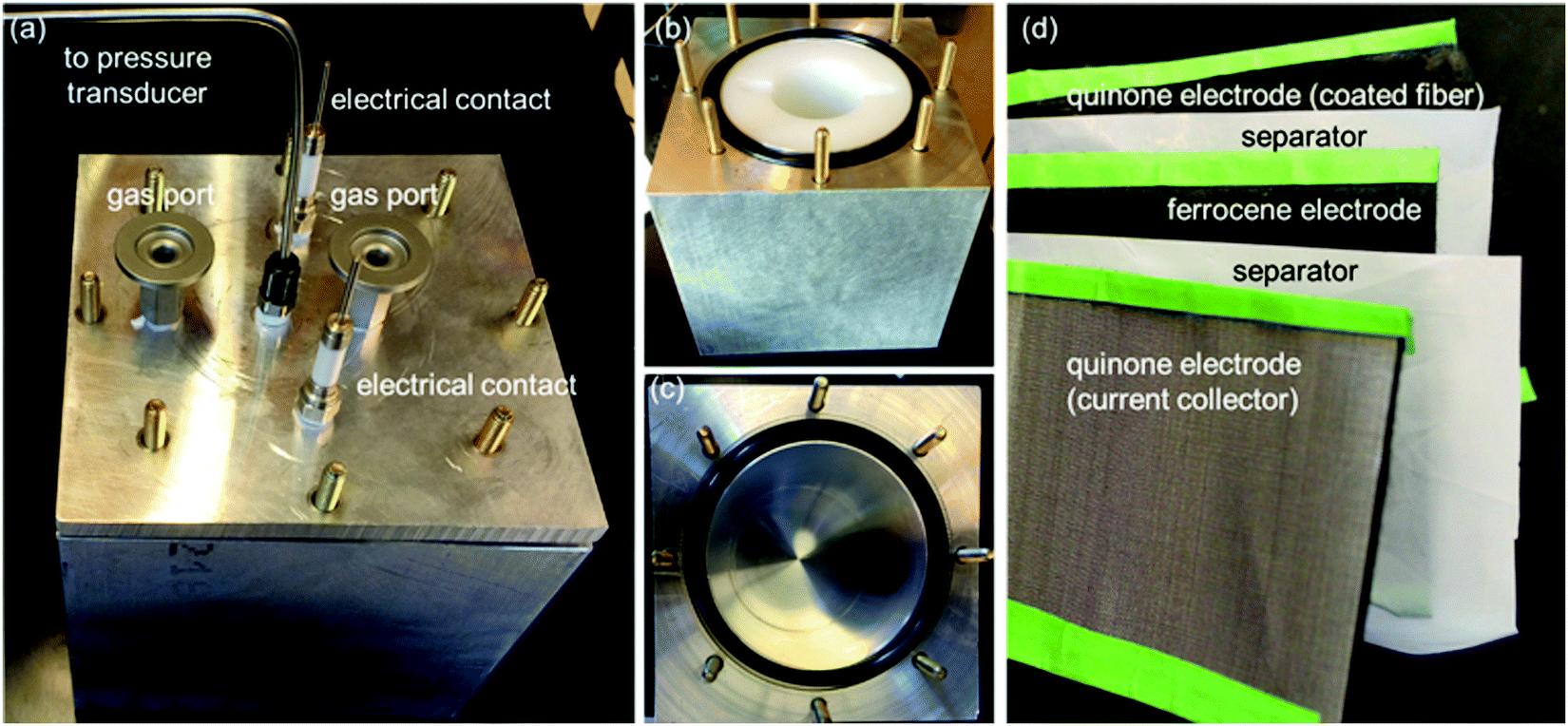
They obviously have a nice machine shop at MIT.
The system shows nice stability over a large number of cycles:
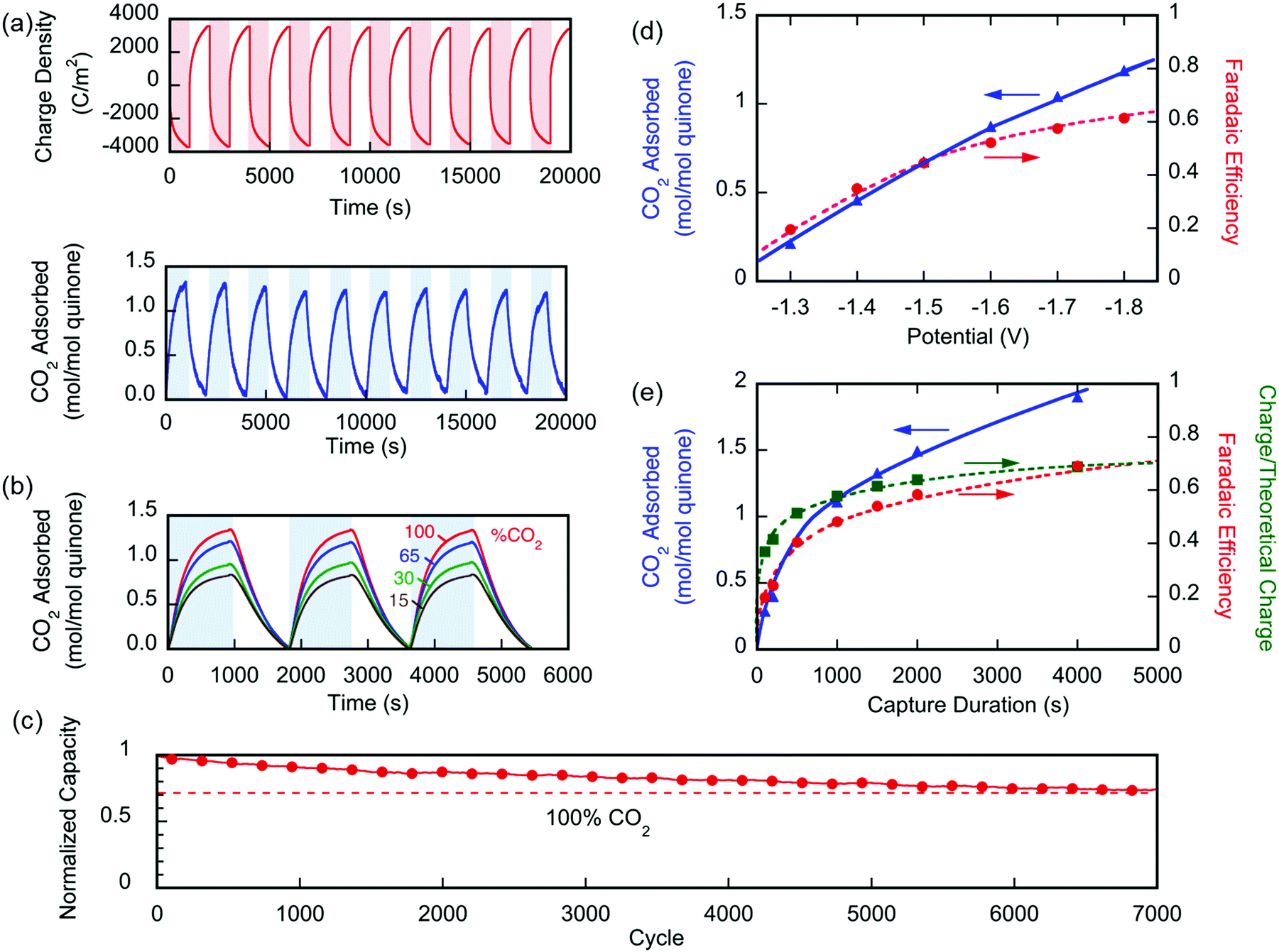
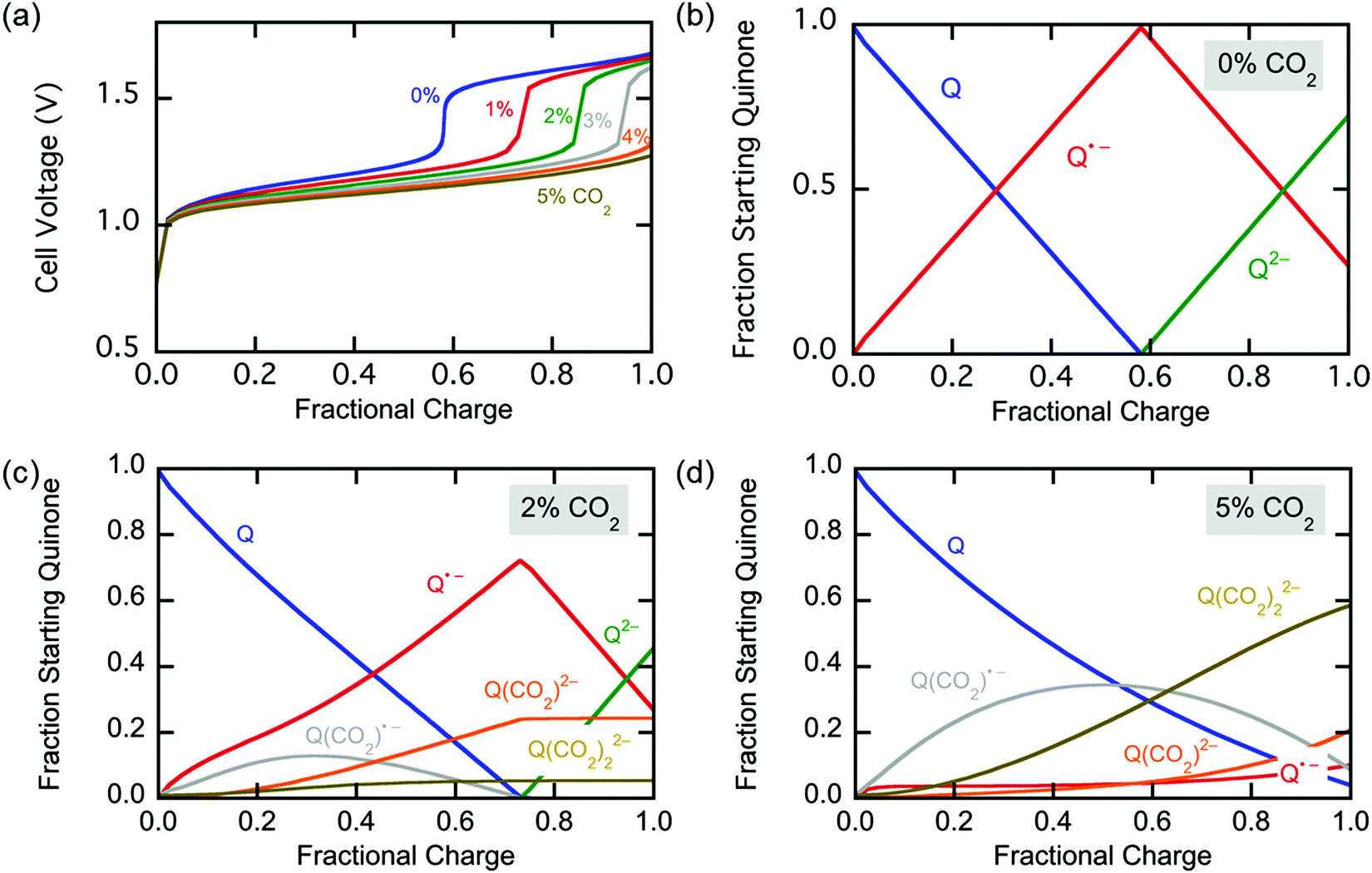
Fig. 6 (a) Changes in the number of moles of CO2 captured upon charging and discharge of the electrochemical cell over 10 cycles, normalized by the moles of quinone on the electrode ( ). The CO2 captured from and released to the chamber tracks the charge applied to the electrochemical cell, normalized by the area of the cell ( ). (b) The CO2 captured under different feed concentrations. (c) Capacity of cell over 7000 cycles. In a different set of experiments using a larger cell and cavity, (d) shows the effect of varying charging potential for a 1000 s capture and (e) shows the effect of varying the capture duration at ?1.8 V capture potential. These experiments were conducted at T ? 21 °C.
A cartoon of the configuration of the system:
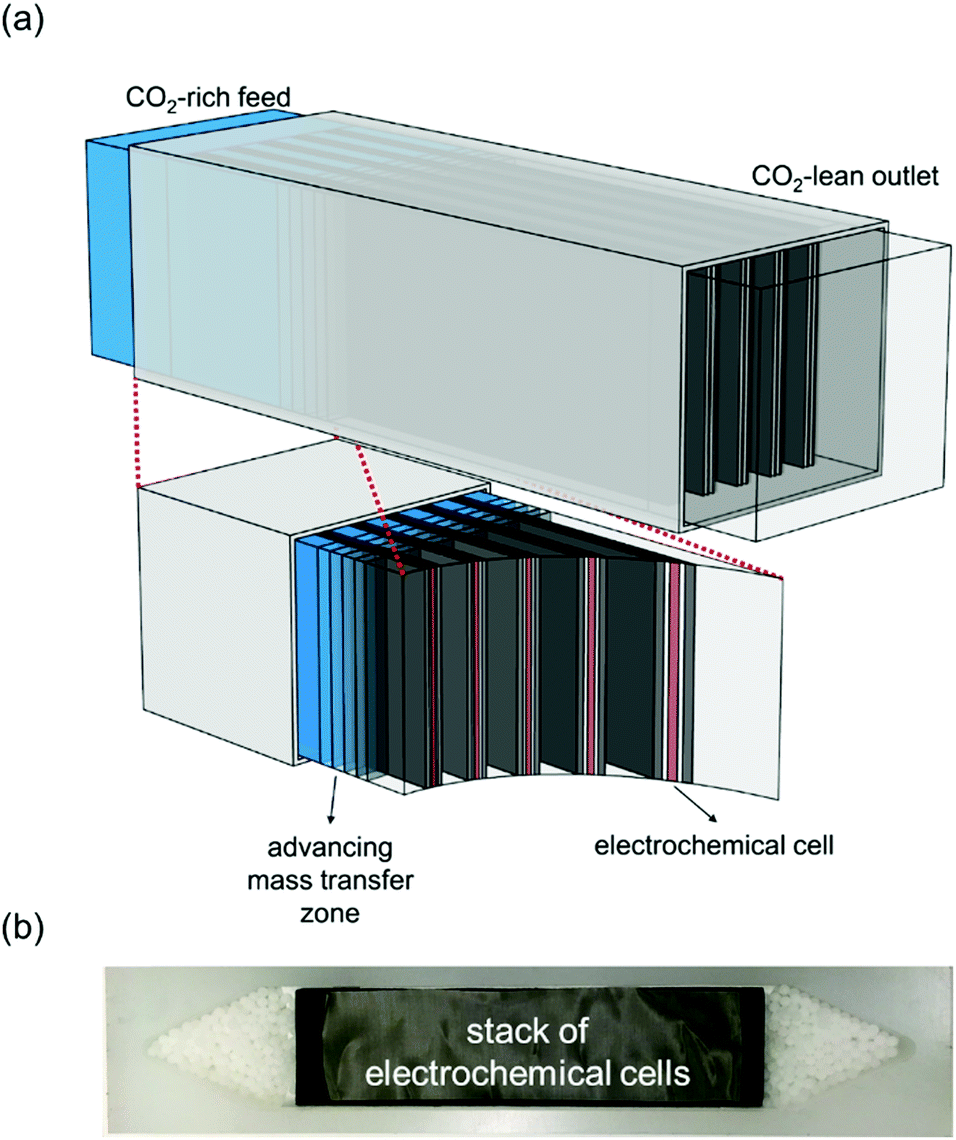
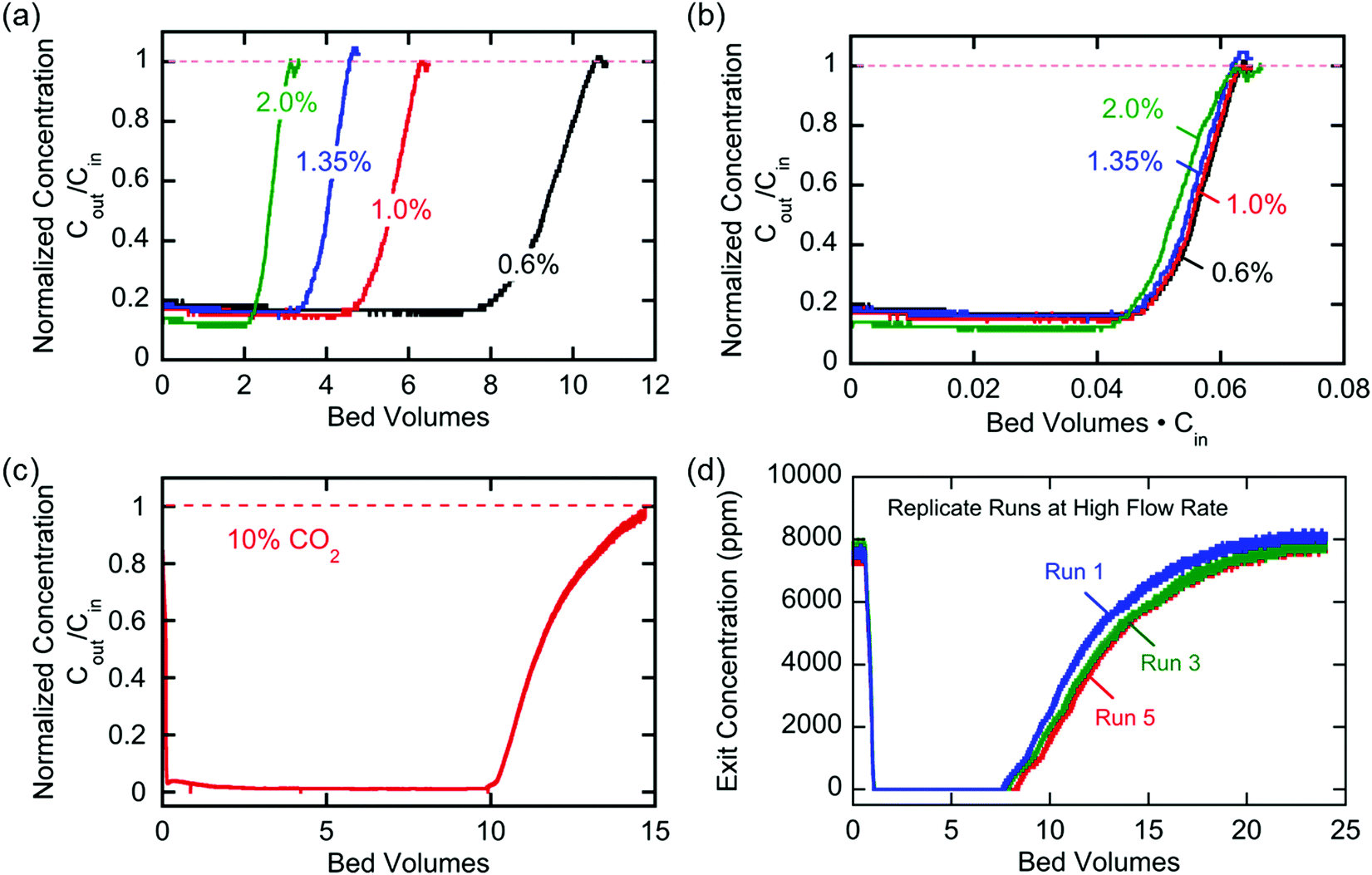
Breakthrough at various concentrations:
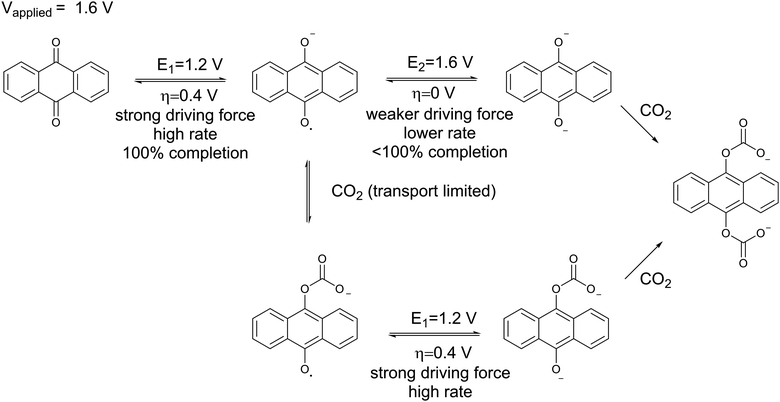
A chemical schematic of the process:
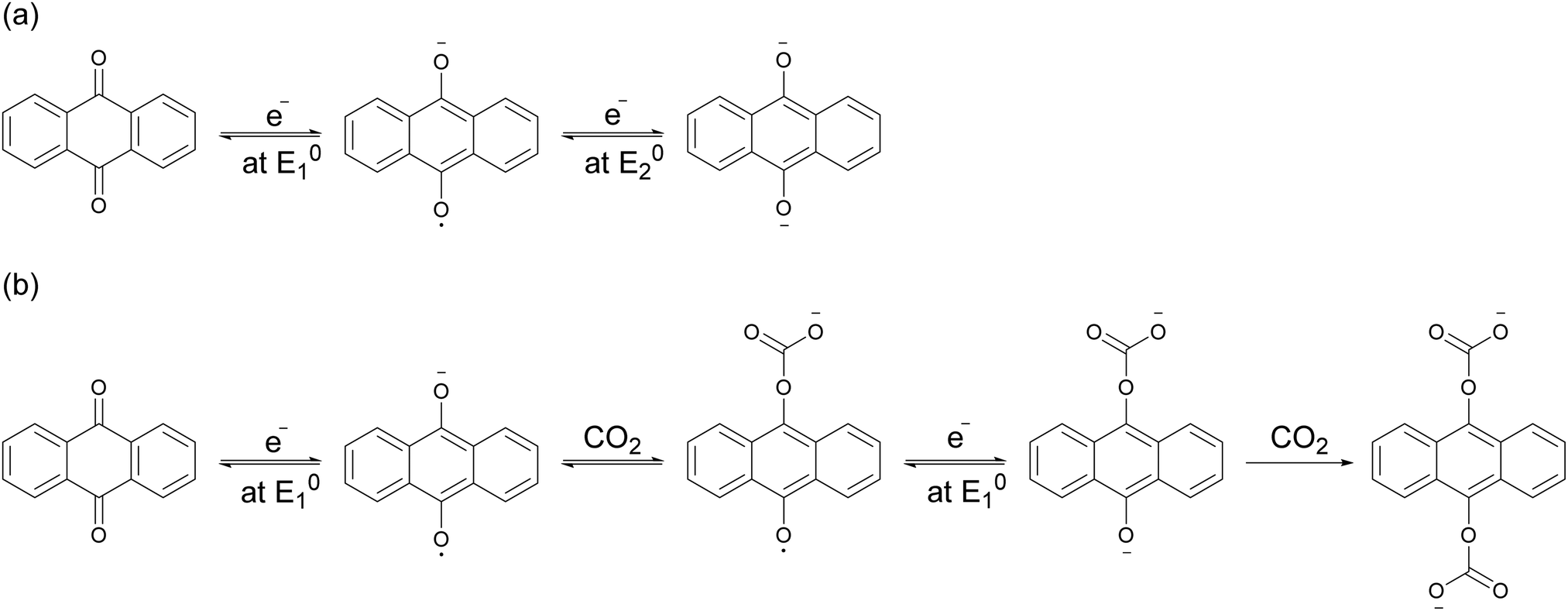
The electrochemical reaction scheme:
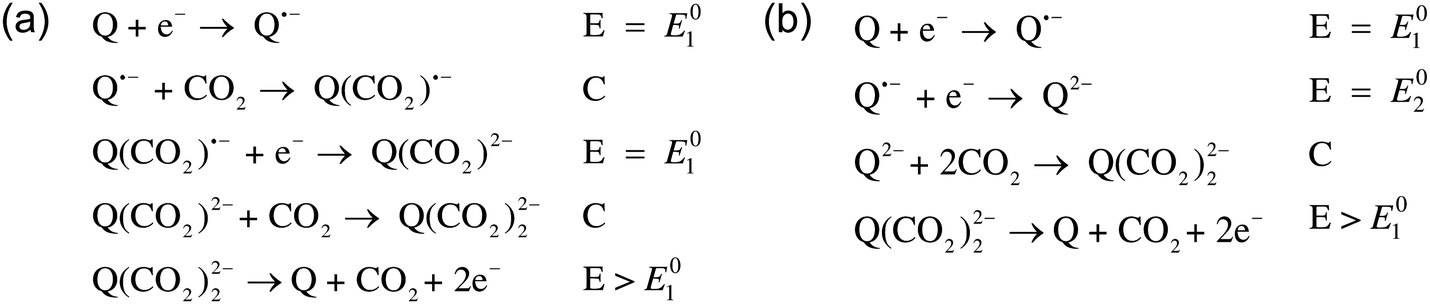
A cartoon of the electrochemical cell configuration:
A graphic of charge and discharge of the system:
More on breakthrough (the physical saturation of the system):
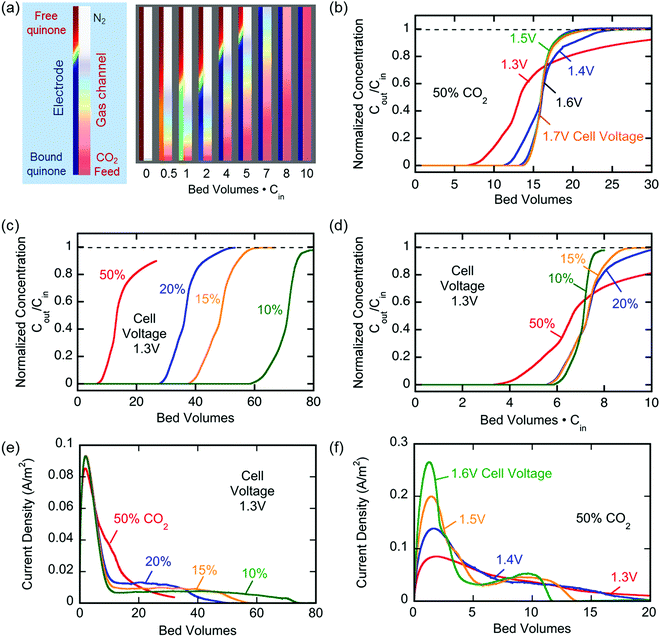
More electrochemical schematics:
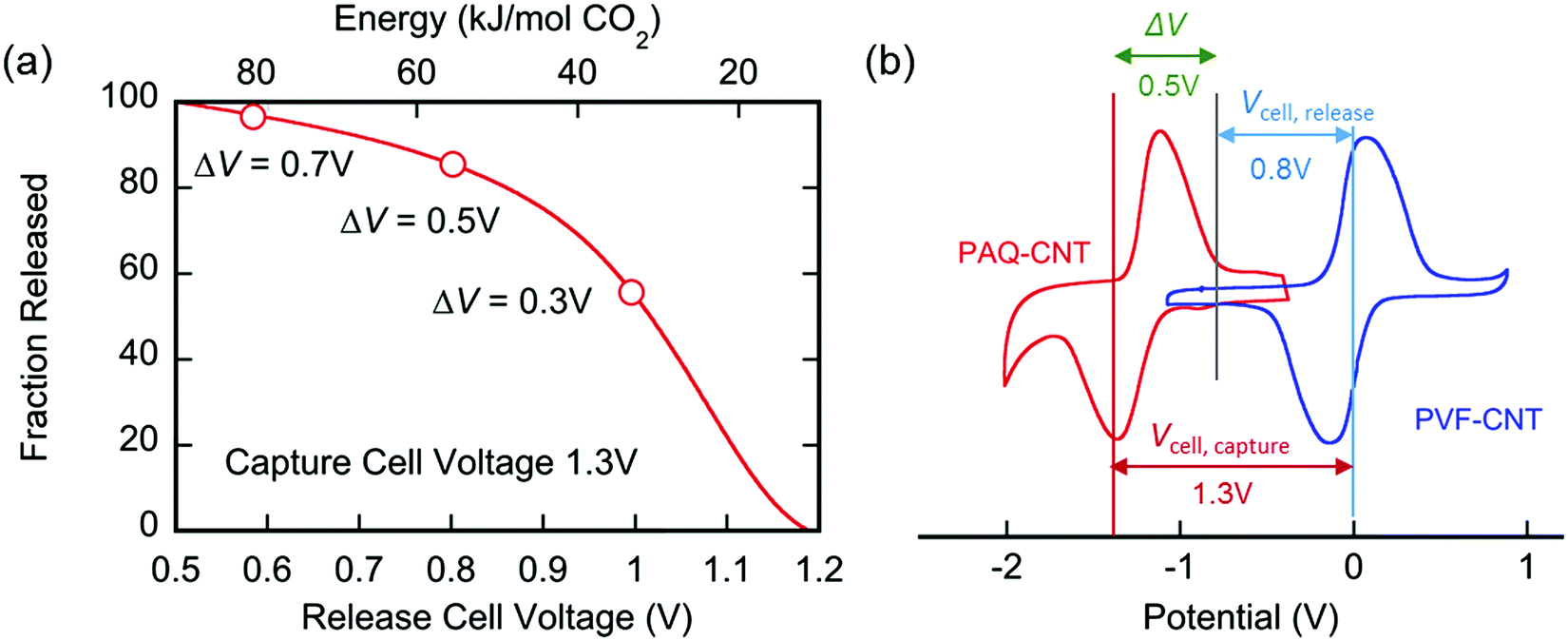
An important graphic showing the energy penalties associated with carbon capture using this device:
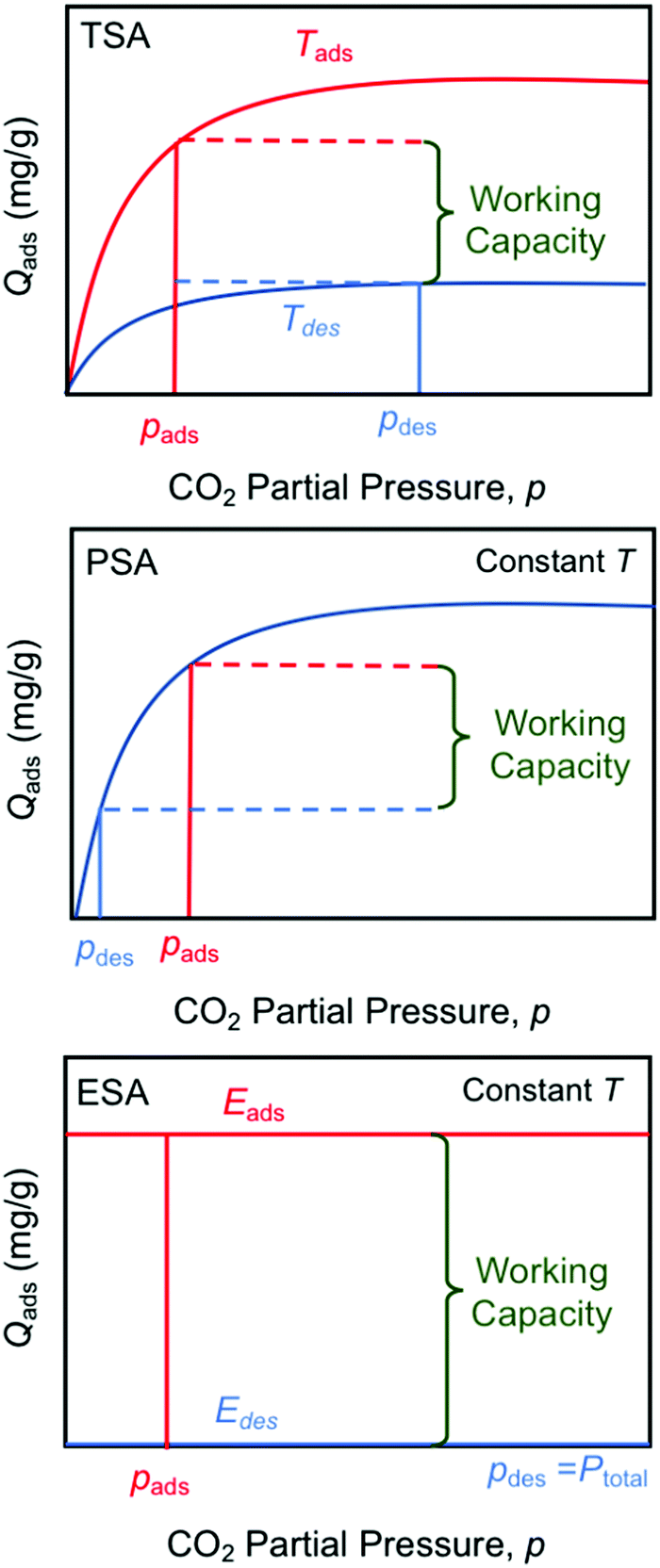
There are several types of "swing" approaches to gas separations commonly used, "temperature swing" - a simple well known example is to use a metal hydroxide, calcium hydroxide ("slaked lime"
They are compared in this graphic:
I note that electricity is always produced at a thermodynamic loss, and thus electricity can, and often is, thermodynamically questionable. Irrespective of popular opinion to the contrary, electricity is not "green" or "clean."
However, there are circumstances where it can be utilized as a thermodynamic enhancer, specifically at very high temperatures, where it is a side product of another process. For example, the thermochemical splitting of water (or carbon dioxide) can be driven at high temperatures with far greater thermodynamic efficiency than via electrochemical approaches, most famously used for water electrolysis. Since the hydrogen and oxygen in the thermochemical water case, and carbon monoxide and oxygen in the carbon dioxide thermochemical case, will ultimately be brought to ambient temperatures, a temperature gradient is necessary, and as such can be utilized to drive turbines (Brayton), boil water (Rankine) or both, raising the efficiency.
Such temperatures are only economically, thermodynamically and environmentally viable with nuclear energy.
Cool idea; cool paper.
Have a nice day tomorrow.
Influence of Sewage Sludge on Ash Fusion during Combustion of Maize Straw
The paper I'll discuss in this post is this one: Influence of Sewage Sludge on Ash Fusion during Combustion of Maize Straw (Liu et al, Energy Fuels 2019, 33, 10, 10237-10246)
As things stand right now there is nothing "green," about the combustion of biomass. Biomass combustion is responsible for slightly less than half of the 7 million air pollution deaths we accept each year without a whimper of protest, although such deaths are largely - but hardly entirely - found in the third world as a result of the combustion of things like straw and garbage indoors in the absence of suitable stoves. The extent to which this practice is "renewable" is a function of the depletion of soils in which the biomass is grown. The real "green revolution" of the 1950's was dependent on the fertilization of soils with fixed nitrogen, which nevertheless a threat to the planetary atmosphere - and, certainly of as much or possibly even greater concern, phosphorous, an essential largely mined resource which is very much subject to depletion.
Despite the above statement it does seem to me that the combustion of biomass under oxyfuel combustion - that is in an atmosphere of pure oxygen - does have much to recommend it. It is entirely possible, it seems to me, to do this in a closed system, one with no material exchange to the environment under any but entirely controlled conditions - which would include useful materials, including relatively pure carbon dioxide available for reduction to solid forms of carbon. Any carbon so obtained would be effectively removed from the atmosphere, and thus the process would not be carbon neutral but rather carbon negative.
I've thought a great deal about such systems, and daydream about them quite frequently, but a purely technical issue is the material nature of the reactors which might do this. Biomass contains a number of inert materials, some of which at high temperatures in the presence of oxygen can be quite corrosive. A material for accomplishing this must therefore able to withstand high temperatures while avoiding corrosion. I believe modern materials science can meet the challenge, but it is in no way a "slam-dunk." Another important issue is heat exchange. Slags can form on the walls of reactors that are difficult to remove, and also prevent free heat exchange, representing an engineering difficulty for the recovery and use of energy in these kinds of processes.
I once read a book called "The Big Necessity" by a woman named Rose George which was a wonderful, um, not exactly "popular" book but written at a level not requiring a scientific education, a rumination on human shit, and by human shit I am not referring to the racist orange thug in the White House, but rather that brown stuff, human feces.
One of the greatest waste disposal on this planet, short only of the problem of dangerous fossil fuel waste, is precisely that, human shit.
Actually though, sewage sludge might well, if regarded correctly, represent a resource, inasmuch as it contains water, carbon, and the aforementioned phosphorous, a very serious matter.
The aforementioned paper points to some possible advantages to including sewage sludge in the combustion of biomass, and it caught my eye.
This excerpt from the introduction to the paper describes in more detail describes some of what I've just said, although I would regard the first two sentences as being highly questionable as practiced:
The ash content of agricultural waste is usually much higher than that of the woody biomass, whereas the composition of ash is also more complex and varied.(6) Agricultural waste contains a large amount of alkali metals (potassium and sodium), as well as related inorganic elements including calcium, magnesium, chlorine, and sulfur.(2,6,7) During the combustion process, most of the potassium in the fuels reacts with silicon to form potassium silicates with a low melting point. These potassium-containing compounds with low melting points exist in a molten state and lead to sintering and slagging at the bottom of the furnace.(6?8) When using a fluidized bed as combustion or a gasification reactor, potassium may also react with the bed material to form low-melting eutectic compounds, which results in the agglomeration of particles, hinders fluidization, and even causes failure of the fluidization.(9?11) Some of the potassium-containing compounds evaporate in the gas phase (such as KOH, KCl, K2CO3, and K2SO4) and condense or deposit on the solid or liquid phase on a low-temperature heating surface, eventually destroying the heating surface.(2,6,8,12) Straw is the most common agricultural waste and has considerable potential for development in terms of combustion for heat and electric power.(13) During the combustion process, the chemical reaction mechanism and the theoretical knowledge of straw (mainly wheat, cotton, and maize) ash have been extensively studied...
....In recent years, a variety of chemical additives have been commonly used in industry to alleviate the problems of ash sintering and slagging. However, this may require high investments and may reduce the economic viability of using these additives in industrial applications.(16,17) Therefore, it is necessary to find a new low-cost, environmentally friendly, anti-slagging additive. The co-combustion of a suitable amount of sewage sludge (SS) and potassium-enriched biomass can alleviate the corrosion of the heating surface, which is a good alternative to using chemical additives.(18?22) SS contains a large amount of silicon, aluminum, phosphorus, iron, and calcium. It was found that SS can capture the alkali metals in the straw and react with it to form high-melting-point compounds, reducing the formation of low-melting-point potassium compounds.(23) Therefore, SS can effectively alleviate the problems of sintering and slagging of biomass ash...
...Li et al.(25) studied the reaction mechanism of phosphorus in SS and potassium in wheat straw. The results showed that the reaction formed high-melting-point potassium aluminosilicate and alkali metal phosphate, which increased the potassium fixation rate of mixed ash. Skoglund et al.(26) conducted a cofiring experiment between biomass and municipal sludge. It was found that the alkali-chloride in biomass ash transformed into alkali metal sulfate after adding SS, which could reduce the risk of alkali metal chloride-related corrosion and slagging.
In general, SS can be used as an anti-slagging additive for the combustion of maize straw (MS), but the scientific evidence for evaluating engineering application feasibility and conducting cost comparison analyses was necessary. The main objective of this work is to study the effect of SS on MS alkali metals’ release characteristics and slagging. The potassium retention rate and the sodium retention rate of MS, SS, and their blends, as well as their slagging characteristics and ash characteristics, were obtained. The results obtained in this experimental study can provide data and theoretical references for sewage sludge’s use as an anti-slagging additive.
This is a Chinese paper and the sewage sludge in this case was dried. (I'm personally not sure that drying the sludge would be a good idea in the long term, as this incurs an energy penalty, but this is their process, not mine.)
They usefully, show the form of the maize straw (MS) and by was of eliminating the puerile silliness and squeamishness associated with this nevertheless important waste form, show a picture of the dried sewage sludge used in their experiments:

The caption:
The maize straw was locally grown:
2.2. Combustion Process. The combustion experiments were conducted in a muffle furnace. The door was kept semiopen to ensure that the sample was completely burnt in the air. The experiments were conducted at temperatures of 700, 800, and 900 °C. When the furnace temperature reached the set value, four samples (MS, M9S1, M8S2, and SS) were sent to the muffle furnace. To ensure the burning of fuel, each experiment lasted 60 min. After this, ash was collected for subsequent analysis.
Table 1:

Table 2:

The raw materials were analyzed by atomic absorption spectroscopy (AAS) after digestion whereas the ash was measured by XRF. In my opinion ICP/MS is the "go to" technology for elemental analysis and is preferred to AAS, but AAS has a long history and is generally satisfactory for use except where very sensitive analysis is required. (ICP/MS might have picked up things like cadmium and lead, the former being a big problem in agriculture, albeit in Southern as opposed to Northern China.) XRF has the advantage of being able to say something about speciation and also the capability of picking up chlorine, a very important element when one is considering issues in corrosion, a topic I may discuss when discussing other papers that have recently caught my eye in the carbon capture via biomass schemata.
This is how the ash looks when prepared at different temperatures:

The caption:
There is a tendency for the alkali metals to migrate during the combustion process, via volatilization. Corrections were applied to reflect the differences between the starting material and the ash.
The following graphics touch on that point and the ability of sewage sludge to mitigate this migration.

The caption:
XRD (X-ray diffraction) analysis of the speciation observed:

The caption:

The caption:
The following graphics refer to the XRD diffraction Energy Dispersive Spectroscopy, where the composition of the marked particles is determined.

The caption:
A table of results:

Similarly:

The caption:

And finally:

The caption:

Some commentary:
The difference in the ash chemical composition is the root cause for the difference in biomass ash melting temperature. The most commonly used indicators for determining the degree of biomass slagging are the ratio of alkali to acid, the ratio of silicon to aluminum, and the ratio of iron to calcium. The alkali acid ratio refers to the ratio of the sum of the alkaline components (oxides of iron, calcium, magnesium, potassium, etc.) to the sum of the acidic components (oxides of silicon, aluminum, and titanium) in the biomass ash. The ratio of silicon to aluminum refers to the ratio of the oxide of silicon to the oxide of aluminum in the biomass ash; the ratio of iron to calcium refers to the ratio of the oxide of iron to the oxide of calcium in the biomass ash.
The paper is interesting because it tells us a great deal about the properties of biomass both in the form of straw and in the form of sewage sludge, the latter being a material that represents a huge environmental problem but also may prove to be an important resource.
This is an air based combustion system, and differs from other alternatives to processing, for example, high temperature steam reforming, or dry (CO2) reforming, and oxyfuel combustion.
The paper does not address the suitability of these ashes for the recovery of phosphorous, for example, and other elements, nor does it specifically address the materials science issues connected with, for example, corrosion and scaling.
Nevertheless, this is very valuable information in defining a path forward for future generations to recover from what we have done to them.
I trust you're enjoying your work week.
Mathematical Modeling of a Microfluidic for the Reduction of Carbon Dioxide.
The paper I'll discuss in this post is this one: Correlating Uncertainties of a CO2 to CO Microfluidic Electrochemical Reactor: A Monte Carlo Simulation (Raman et al, Ind. Eng. Chem. Res.2019, 58. 42, 19361-19376) It's in the current issue of this journal as of this writing.
The paper's introductory graphic is a cartoon evoking an Ishikawa "fish plot" diagram:

Removal of the dangerous fossil fuel waste carbon dioxide, which is killing the planet, from the atmosphere is only possible if future generations have something to do with it. Although our current practice is to burn a dangerous greenhouse gas, dangerous natural gas; a liquid, dangerous petroleum; or a solid, dangerous coal, to produce this dangerous fossil fuel waste, with sufficient energy, the combustion of these dangerous fossil fuels is reversible, via a reaction known as the Boudouard Reaction.
I've written at length in various places about this reaction.
Here, from another paper about adjusting the thermal equilibrium the reaction describes, is a graphic that describes the Boudouard reaction:

Source: Microwave-Specific Enhancement of the Carbon–Carbon Dioxide (Boudouard) Reaction
The reaction is shown at the top of this graphic. The equilibrium lines in this graphic, one is thermal and the other driven by microwave radiation, shows that one can make carbon - which under the right conditions can be processed into useful materials - from carbon monoxide if one removes one of the reactants, which would be carbon dioxide.
A great deal has been written in the scientific literature about reducing carbon dioxide to carbon monoxide, and this paper is just one example. Microfluidic devices are just what they sound like, devices with small channels that are designed to maximize surface area by forcing a fluid - in this case as gas, the dangerous fossil fuel waste carbon dioxide - through tiny channels. Although the technology for making these devices has advanced to a high level only in recent times, these types of devices have long been known: A well understood microfluidic device, in this case for fluid exchange, is a human lung.
There are many variations on the Boudouard reaction, some of my personal favorites being "dry reforming" of waste organic materials, municipal and industrial carbon based wastes for example, or the dry reforming of biomass. The method described here is electrochemical. Although electricity is a thermodynamically questionable approach to energy storage or materials processing, there are certain conditions where grid and load balancing make waste electricity available for electrochemical processes, for example in the case of a plant designed for continuous operation that runs during low load periods.
From the paper's introduction:
Typically, the selectivity toward one or more of the above-mentioned products is controlled by the choice of the cathode side catalyst including metal surfaces,(21) metal nanoparticles,(22) metal oxides,(23) organometallic molecules,(24) and metal and covalent organic frameworks.(25,26) Several recent reviews outline the recent trends in the selectivity-based electrocatalyst development.(27?30) These electrocatalysts are usually studied and screened in a three-electrode setup or an H-cell. However, these reactor configurations can be mass-transport-limited.(31,32) In addition, these systems are batch reactors and are not scalable, making them less relevant for commercialization.
To overcome mass transport limitations and to achieve scalability, flow cell architectures were investigated. These flow cell reactors are of different types, viz., solid oxide electrolysis cells,(33,34) membrane-based electrolytic cell,s(32,35) and microfluidic flow cells (MFCs).(36,37) Berlinguette and co-workers(38) present a detailed account on the development of flow cells for the electroreduction of CO2. Bevilacqua et al.(39) discuss the efforts to scale up these flow cells. Despite a large number of such studies being experimental, there has also been recent interest toward the mathematical modeling of such systems.(6,40?43) These mathematical models, depending on their complexity, can shed light on the intricate interplay between gas transport and electrochemistry. Along with suitable experiments, through these models, we can delve deeply into the effects of various design, physical, material, operating, and electrochemical parameters on the functioning of the reactor...
...during the large-scale fabrication of these microfluidic reactors, the properties may deviate from the values specified for a desired output. Therefore, in practical applications, it is difficult to identify these uncertainties and estimate their influence on the conversion efficiency, reactor performance, and selectivity.
To capture this random yet probabilistic nature of variation of the input parameters, a stochastic method such as Monte Carlo simulations (MCS) is necessary. Through MCS, we can identify not only the most critical input parameter in a given range of operating parameters but also uncover the effects of the simultaneous variation of different input parameters on the system. Following this stochastic approach, deterministic analyses such as identifying optimal regions of operation, different choices of materials, and robust control strategies, diagnoses, and prognoses can be carried out...
...In light of the lack of a stochastic technique that can record the probabilistic nature of the input parameters and their relative impact on the current density and the trade-off between cell performance and conversion efficiency and the Faradaic efficiency of an MFC reactor, first we conduct MCS of a 2D mechanistic model of the MFC reactor. To achieve this, we generate a large random population of input parameters and simulate a detailed mechanistic model of the MFC reactor across the parameter space. A fish-bone diagram relating these stochastic input parameters to the response variables is illustrated in Figure 1. The varied stochastic parameters can be classified as (a) geometric/design, consisting of the thickness of each functional layer and the cell length and width; (b) physical, involving the porosity of the functional layers and the dynamic viscosity of the feed gas; (c) material, consisting of the electrical conductivity of the different functional layers and the ionic conductivity of the electrolyte; (d) operating, including the applied cell potential, temperature, feed gas flow rates, and inlet feed mole fractions; and (e) electrochemical parameters, including the exchange current densities and charge transfer coefficients...
Figure 1:

The caption:
After some following discussion the authors write this to describe their approach to modeling putative electrochemical devices for reducing carbon dioxide to carbon monoxide:
Figure 2:

The caption:
The reactions considered here by the authors involve a hydrogen side product. There are examples of such reactions which do not involve hydrogen, although in the electrical case, the reduced carbon dioxide is made into hydrocarbons and/or alcohols.
From the text:

The oxygen evolution reaction (OER) on the anode side is given as

The latter reaction, the oxygen evolution reaction at an electrode is the subject of much discussion in the scientific literature, because of its nature as a 4 electron reaction. Although electrolysis is well known and often practiced, although most of the world's hydrogen is made by reforming dangerous natural gas, it places limits on the energy efficiency of electrochemical water splitting and is thus open to improvement.
A table of the variables considered.

Some other graphics from the the paper which may or may not mean much:

The caption:
Figure 3. (a) Polarization curve and (b) conversion efficiency (—) and Faradaic efficiency (---) as a function of the applied cell potential, corresponding to the mean values of input parameters.

The caption:
Figure 4. Sample distribution of the inlet CO2 mole fraction, xCO2in (bars), and the fitted normal distribution functions (lines) for different sample sizes: (a) 170 and (b) 103.

The caption:
Figure 5. Ranking of stochastic parameters under the IND scenario at Ecell = ?2.7 V (black), ?2.8 V (blue), ?2.9 V (green), ?3.0 V (gray), and ?3.1 V (violet) for (a) cell performance, (b) conversion efficiency, and (c) Faradaic efficiency.

The caption:

The caption:

The caption:

The caption:
In the above, SIM, IND, and REG refer to manner the Monte Carlo Simulation is run, i.e. the process and sequence by which the variables are subject to perturbations.
This kind of research can be obscure and arcane, but it is very, very, very important nonetheless.
A word we hear too much is could, but I'll use it anyway. Properly focused we could do so much with the power of our scientific tools, but regrettably we are doing very little.
Have a nice weekend.
Bacterial biodiversity drives the evolution of CRISPR-based phage resistance
The paper I'll discuss in this brief post is this one: Bacterial biodiversity drives the evolution of CRISPR-based phage resistance (Ellinor O. Alseth, Elizabeth Pursey, Adela M. Luján, Isobel McLeod, Clare Rollie & Edze R. Westra, Nature 574, 549–552 (2019))
(The authors appear to be 100% women, nice to see.)
CRISPR is very much in the scientific news these days, both as a research tool and as a possible therapeutic agent for a host of genetic diseases, including (but hardly limited to) cancer, which may be thought of as a somatic genetic disease. With respect to its use as a research tool, a few days back I posted in this space, a report utilizing CRISPR to interrogate the toxin resistance that is observed in the Monarch butterfly. Genome editing retraces the evolution of toxin resistance in the monarch butterfly.
I haven't really paid much attention to the nuts and bolts of CRISPR technology, at least until a chance conversation at a science oriented social event stimulated me to do so. I was of course, aware of its role in gene therapy, but not of the basic science underlying it. The appearance of two papers in two subsequent issues of Nature stimulated more interest in the origins and use of this technology.
As many people know, among the many things we are leaving for future generations besides an atmosphere destroyed by appeals to denial, mysticism, fear and ignorance, and effective depletion of many of the elements in the periodic table, is a plethora of dangerous antibiotic resistant bacteria. One avenue for addressing this resistant bacteria is to appeal to an old idea, viral antibiotics, inoculating people with viruses that are known to attack and kill bacteria, viruses know as phages. (Phages are also widely used as research and production tools, particularly for the insertion of genes into organisms in the biotech industry.) This old idea is worth a look given that our understanding of molecular biology has entered a golden age which, one hopes, will be maintained despite the rise of anti-intellectualism on both political extremes.
Anyway.
The CRISPR/CAS-9 system is actually the immune system for bacteria, and this paper is about how this system is utilized to develop resistance to phages.
From the abstract of the paper:
From the introduction:
The authors used in their experiments, a streptomycin resistant strain (PA14) of the P. aeruginosa pathogenic organism and cutlured it in the presence of three other pathogenic bacterial species that are not infected by the DMS3vir virus, Staphylococcus aureus, Burkholderia cenocepacia and Acinetobacter baumannii.
Some pictures from the paper suggesting the results:
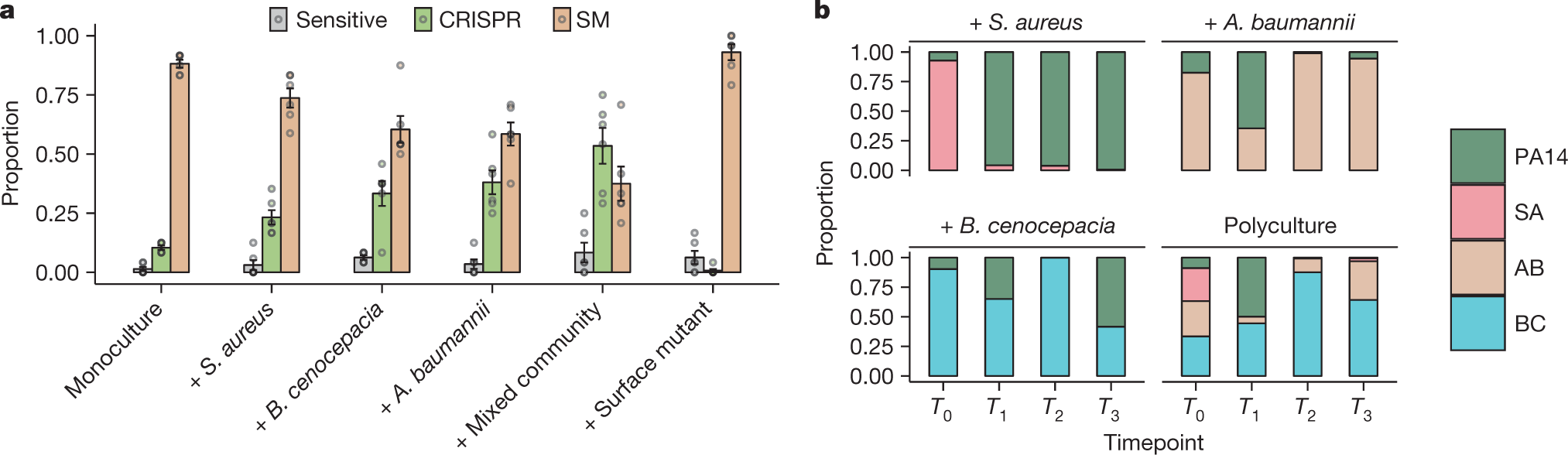
The caption:
The authors also evaluated the potential pathogenic implications of this result by growing cultures in synthetic sputum.
The rise of CRISPR resistance, as shown in the previous graphic in P. aeruginosa in the presence of bacterial diversity is clearly observed.
Another graphic:
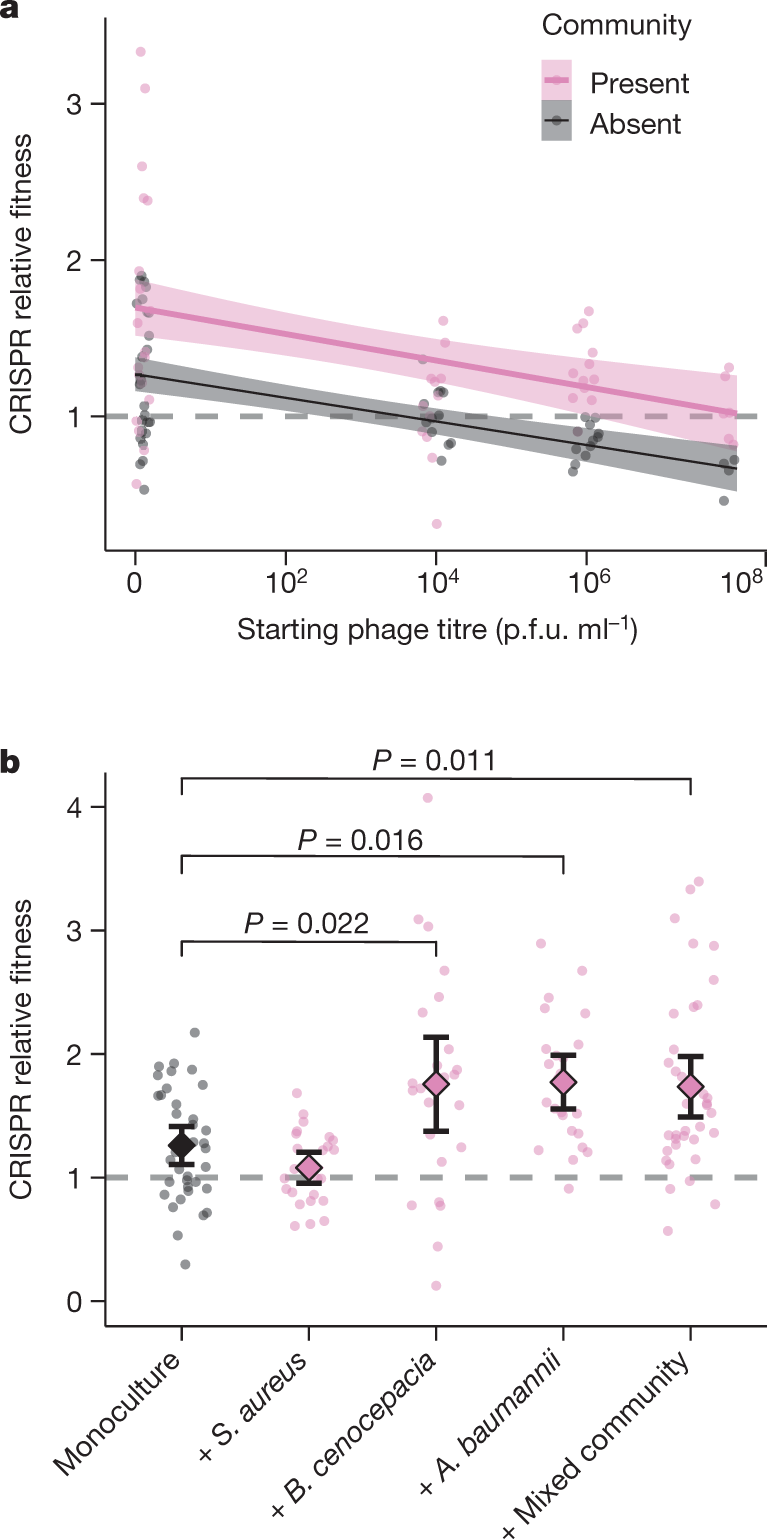
The caption:
Another graphic touching on virulence:
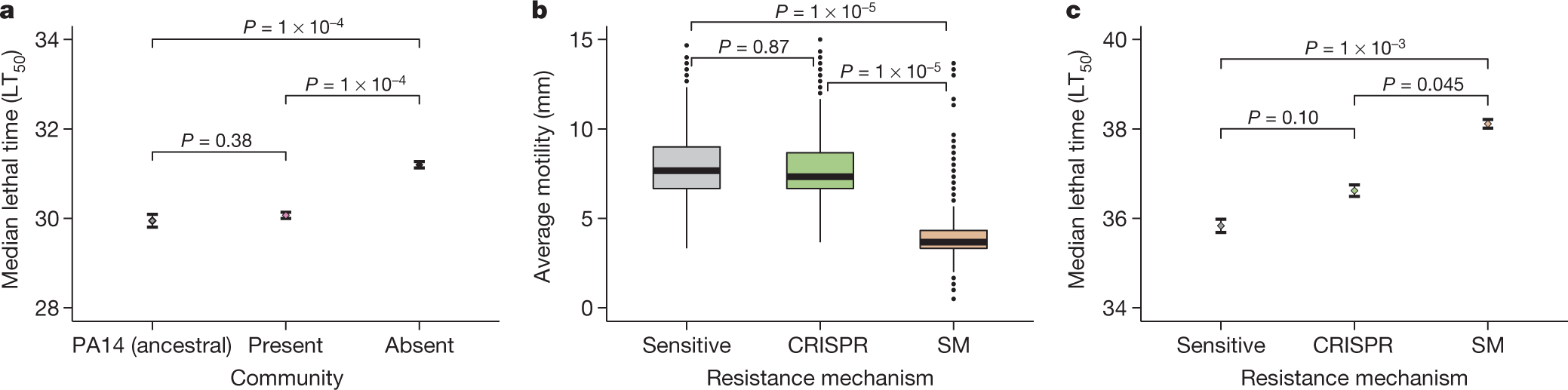
Some excerpts from the concluding discussion:
...Primarily, the absence of detectable trade-offs between CRISPR-based resistance and virulence, as opposed to when bacteria evolve surface-based resistance, suggests that the evolution of CRISPR-based resistance can ultimately influence the severity of disease. Moreover, the evolution of CRISPR-based resistance can drive more rapid phage extinction29, and may in a multi-phage environment result in altered patterns of cross-resistance evolution compared with surface-based resistance30. The identification of the drivers and consequences of CRISPR-resistance evolution might help to improve our ability to predict and manipulate the outcome of bacteria–phage interactions in both natural and clinical settings.
Interesting, I think, and important.
Have a nice day tomorrow.
Genome editing retraces the evolution of toxin resistance in the monarch butterfly.
The paper I'll discuss in this post is this one: Genome editing retraces the evolution of toxin resistance in the monarch butterfly. (Whiteman et al, Nature 574, 409–412 (2019))
One of the happiest memories of the childhood of my sons was when we went to a local park, the New Jersey side of Washington's Crossing Park, where the ranger showed us how to collect monarch butterfly eggs, which were laid on the underside of milkweed plants, poisonous plants that grow wild all around here. We took the leaves home, put them in a butterfly cage, and ultimately the eggs hatched, and caterpillars began munching the leaves. We keep collecting them from the fields around here, until they formed cocoons, pupae, and finally emerged as butterflies, which we released.
It was a beautiful, wonderful experience, and probably one I would have never had were I not a father.
The Monarch's don't really "migrate" as individuals; each year several generations make their way across North America from Mexico, breeding repeatedly, happily munching toxic milkweed all across America.
A truly wondrous life form!
CRISPR/CAS-9 is a gene editing tool developed by Jennifer Doudna and Emmanuelle Charpentier that utilizes a bacterial protein, known as CAS-9, which is in a sense an "immunity defense" for procaryotic organisms, that operates in conjunction with a guide RNA sequence that acts much like "interfering RNA," "iRNA" relying on complementary. By appropriate editing of the RNA sequence, this system can be modified for the purpose of gene editing, both for research purposes and, perhaps, for therapeutic modalities.
(Having participated in my career in a number of "really hot" biomedical fads, I tend to be more "wait and see" than over the top enthusiastic for these sweeping claims.)
The paper cited above is using CRISPR/Cas-9 as a research tool to discover how the Monarch butterfly became immune to the plant toxins in milkweed. This toxicity by the way, protects the Monarch from predators, since the butterflies themselves are toxic.
From the abstract:
Published: 02 October 2019
Genome editing retraces the evolution of toxin resistance in the monarch butterfly
Marianthi Karageorgi, Simon C. Groen, Fidan Sumbul, Julianne N. Pelaez, Kirsten I. Verster, Jessica M. Aguilar, Amy P. Hastings, Susan L. Bernstein, Teruyuki Matsunaga, Michael Astourian, Geno Guerra, Felix Rico, Susanne Dobler, Anurag A. Agrawal & Noah K. Whiteman
Nature volume 574, pages409–412 (2019) | Download Citation
Abstract
Identifying the genetic mechanisms of adaptation requires the elucidation of links between the evolution of DNA sequence, phenotype, and fitness1. Convergent evolution can be used as a guide to identify candidate mutations that underlie adaptive traits2,3,4, and new genome editing technology is facilitating functional validation of these mutations in whole organisms1,5. We combined these approaches to study a classic case of convergence in insects from six orders, including the monarch butterfly (Danaus plexippus), that have independently evolved to colonize plants that produce cardiac glycoside toxins6,7,8,9,10,11. Many of these insects evolved parallel amino acid substitutions in the ?-subunit (ATP? ) of the sodium pump (Na+/K+-ATPase)7,8,9,10,11, the physiological target of cardiac glycosides12. Here we describe mutational paths involving three repeatedly changing amino acid sites (111, 119 and 122) in ATP? that are associated with cardiac glycoside specialization13,14. We then performed CRISPR–Cas9 base editing on the native Atp? gene in Drosophila melanogaster flies and retraced the mutational path taken across the monarch lineage11,15. We show in vivo, in vitro and in silico that the path conferred resistance and target-site insensitivity to cardiac glycosides16, culminating in triple mutant ‘monarch flies’ that were as insensitive to cardiac glycosides as monarch butterflies. ‘Monarch flies’ retained small amounts of cardiac glycosides through metamorphosis, a trait that has been optimized in monarch butterflies to deter predators17,18,19. The order in which the substitutions evolved was explained by amelioration of antagonistic pleiotropy through epistasis13,14,20,21,22. Our study illuminates how the monarch butterfly evolved resistance to a class of plant toxins, eventually becoming unpalatable, and changing the nature of species interactions within ecological communities2,6,7,8,9,10,11,15,17,18,19.
An excerpt of the introductory text:
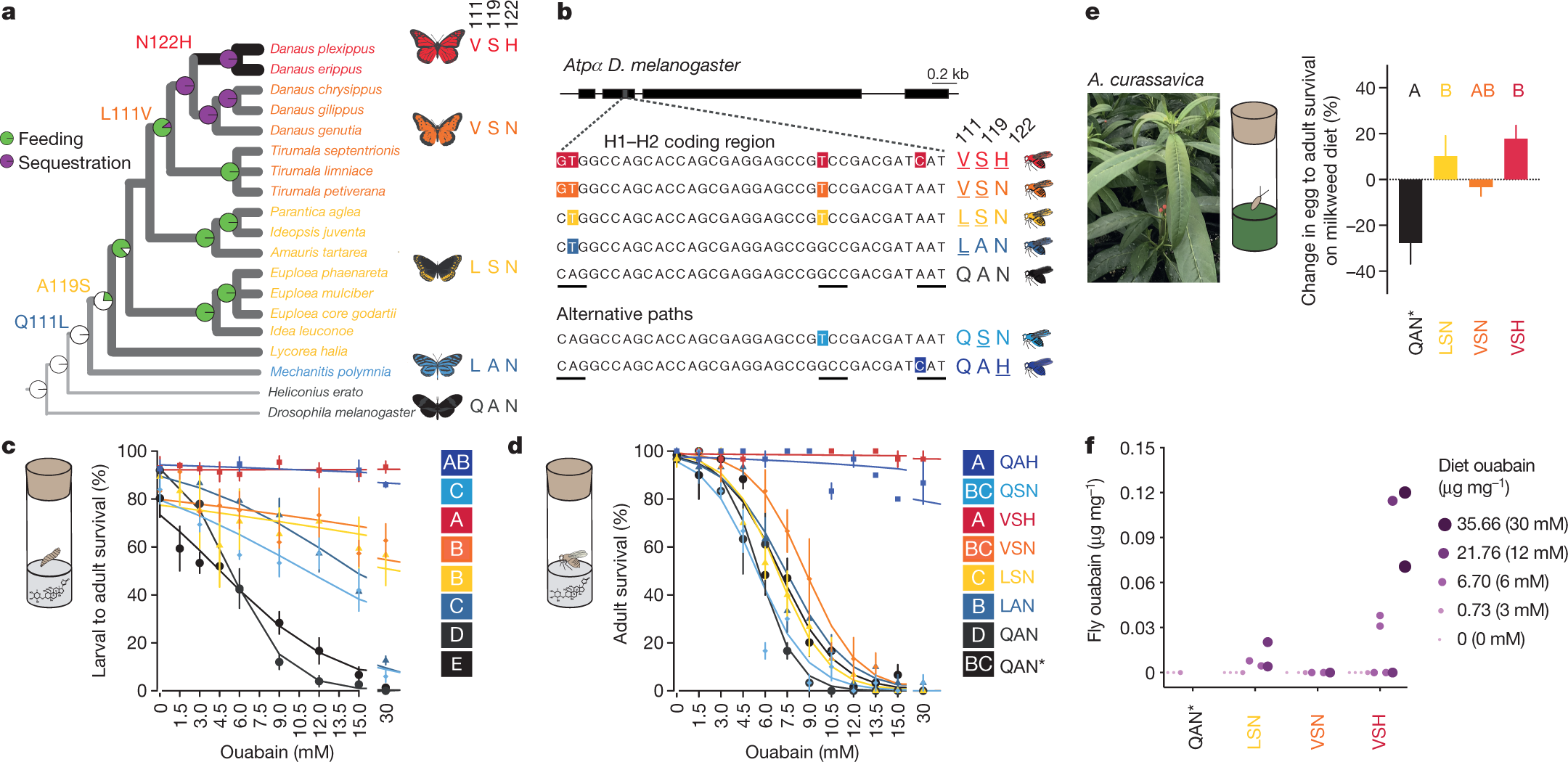
We have identified a core set of amino acid substitutions in cardiac glycoside-specialized insects that define potential mutational paths to resistance and TSI. We focused on the first extracellular loop (H1–H2) of ATP?, where most candidate TSI-conferring substitutions occur7,8,9,10,11 (Fig. 1a). We used maximum likelihood to reconstruct ancestral states for cardiac glycoside specialization (feeding and sequestering) and amino acids within the H1–H2 loop of ATP? across a species phylogeny...
The authors identified a series of known amino acid substitutions in the ATP? in the Monarch, at residues 111, 119, and 122.
A figure from the text:
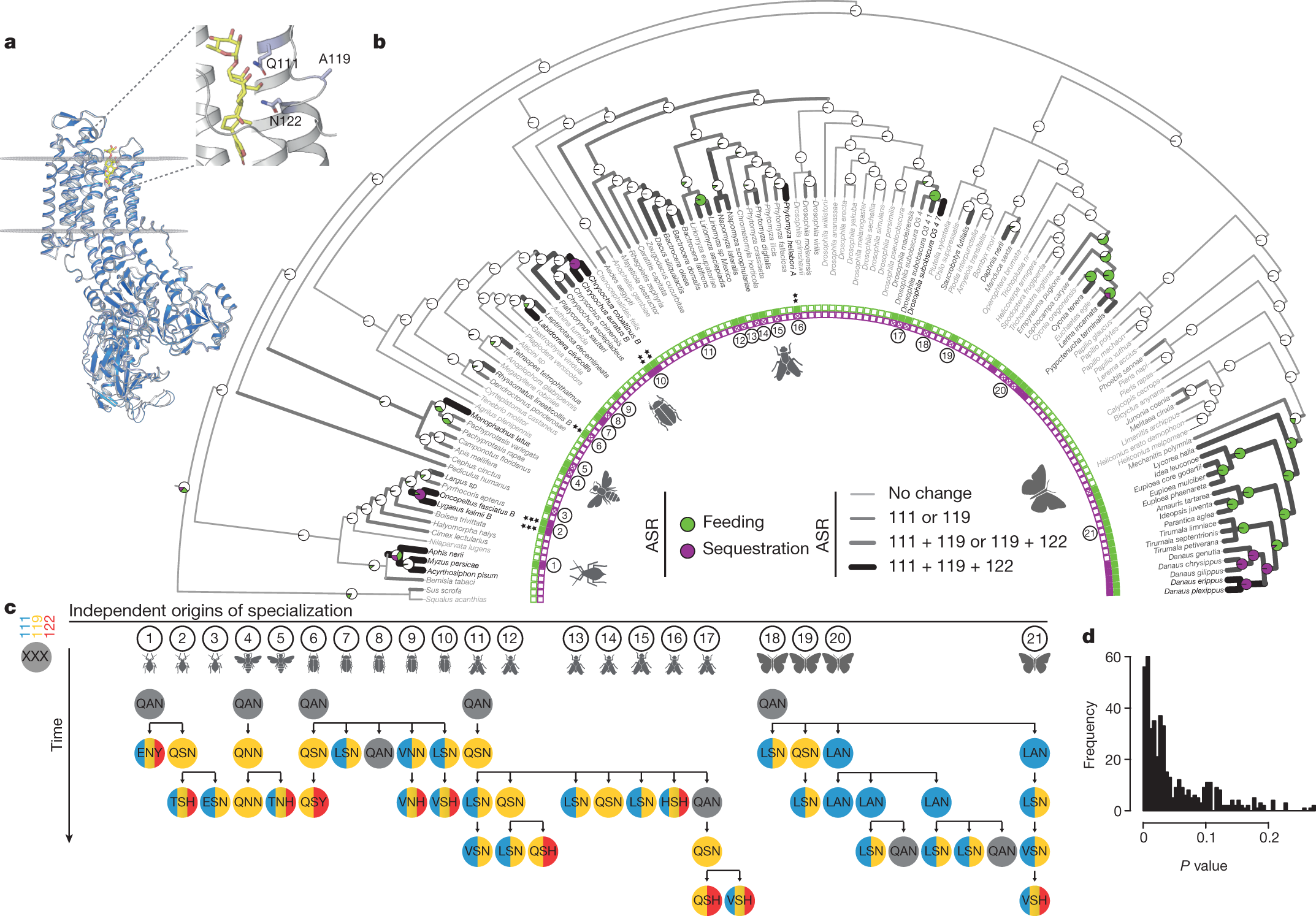
The caption:
The DNA of the Drosophilia fly was modified via editing to provide the necessary homology allowing the organisms to feed on cardiac glycosides as well.
Another figure from the paper:
Some observations:
When knock-in line eggs were placed on medium containing the suite of cardiac glycosides found in the leaves of the milkweed species Asclepias curassavica and A. fascicularis6, monarch lineage fly genotypes generally showed increased egg–pupal and egg–adult survival rates (Fig. 2e, Extended Data Fig. 7), although not always for VSN (Extended Data Figs. 3, 7). The LSN, VSN and VSH genotypes may enable insects to cope with the complex milieu of cardiac glycosides encountered during host shifts to these plants.
The monarch butterfly ATP? substitutions at positions 111, 119 and 122 may unlock a passive evolutionary route to cardiac glycoside sequestration, as we found small amounts of ouabain in newly emerged adult ‘monarch flies’ reared as larvae on a diet containing ouabain (Fig. 2f). However, toxin concentrations were far lower than in monarch butterflies, and the location of ouabain in flies is unclear6,17,18.
Another graphic:
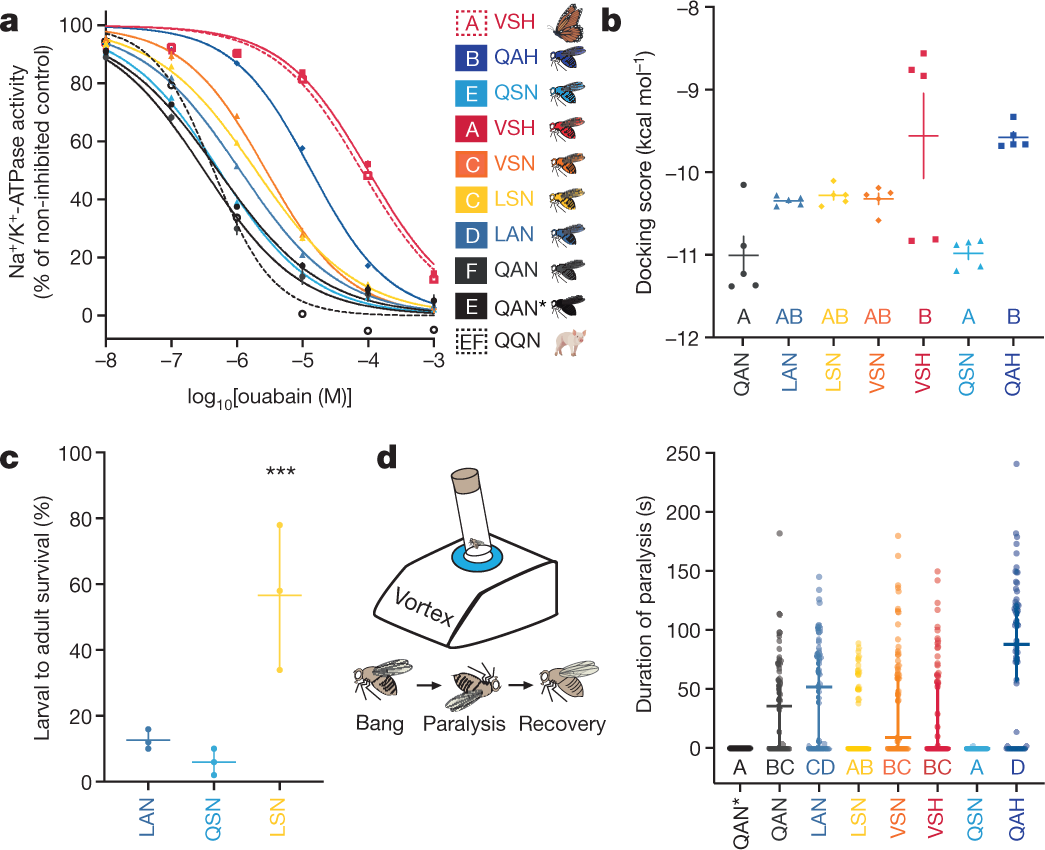
The caption:
The full paper makes comments about the evolutionary pathway by which the TSI, Target substance insensitivity, to cardiac glycosides evolved.
(The old drug digitalis and related digoxin fit into this class of compounds.)
Some concluding remarks:
This technology is very powerful.
Like all powerful technologies, it has a potential to do good and great things, and likewise, bad and terrible things. The choice is moral. As old and as cynical as I am, I still believe, in spite of it all, in the capacity for humanity to come down on the side of good and great.
Have a nice day tomorrow.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512