NNadir
NNadir's JournalUtilizing the 21 Tesla Magnet at the National High Magnetic Field Lab to Characterize Asphaltenes.
The papers I'll discuss in this post are the two parts of papers appearing recently in the scientific journal Energy and Fuels.
They are: Probing Aggregation Tendencies in Asphaltenes by Gel Permeation Chromatography. Part 1: Online Inductively Coupled Plasma Mass Spectrometry and Offline Fourier Transform Ion Cyclotron Resonance Mass Spectrometry (Marshall et al., Energy and Fuels, Energy Fuels 2020, 34, 7, 8308–8315)...
...and...
Probing Aggregation Tendencies in Asphaltenes by Gel Permeation Chromatography. Part 2: Online Detection by Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Inductively Coupled Plasma Mass Spectrometry (Marshall et al., Energy and Fuels, Energy Fuels 2020, 34, 9, 10915–10925).
This journal's papers are generally overwhelmingly about dangerous fossil fuels. Anyone with a passive knowledge of my often turgid writings will be aware that I oppose the use of all dangerous fossil fuels and believe they must be phased out as quickly as possible on an emergency basis. I nonetheless regularly read this journal for several reasons. One is that while I favor largely doing away with the car CULTure - something of a hard sell I freely admit - there are certain materials, including to be perfectly honest, fuels, that cannot be ethically banned while respecting human development goals, that are obtained from dangerous fossil fuels. Thus to maintain access to these materials while simultaneously banning the mining of dangerous fossil fuels, we must understand what these materials are and how they can be either manufactured or replaced without the use of dangerous fossil fuels themselves. Of particular importance are "cokes" which are carbonaceous materials used widely in the reduction of metal ores, either in thermal settings (as in Bessamer furnaces in the steel industry) or as electrodes in Hall Heroult and FFC processes. The second reason is that many papers, especially those in the (generally smaller) section related to biomass offer insights to the now necessary goal of removing carbon dioxide from the air. A third reason is that there is generally a section in this journal connected with the capture of carbon dioxide. Although these are largely addressed to the quixotic idea of giant underground dangerous fossil fuel waste dumps (aka "sequestering" ) they are also relevant to more sane means of addressing climate change. A final reason is that often these papers just contain good science.
The papers under discussion here are largely directed to problems in the dangerous fossil fuel industry, in particular, the dangerous petroleum industry, but they are relevant actually to many of the reasons I gave above. For example, in the case of removing carbon dioxide from the air: In the high temperature reformation of biomass, it is often the case that "tars" are formed; these are in fact, asphaltenes, close to those found in dangerous crude petroleum. Although asphaltenes are problematic - very problematic - in industrial equipment, they are widely used as the familiar product asphalt, generally an aggregate of sand and asphaltenes. Thus, were we to pave roads, bicycle paths and walkways with asphaltenes obtained from the reformation of biomass, we would be removing carbon dioxide from the air and effectively sequestering in an economically viable manner. Indeed, as we will see below, asphaltenes can be regarded, in part, as fragmented graphene, and a deeper understanding of their chemistry can lead to new insights in materials science. Finally this paper utilizes one of the tremendous resources built in an era when the US government was more committed to science rather than racism, corruption, lies, hypocrisy, the subjugation and denigration of women, power grabbing and the spreading of diseases as it is today in the Senate and Administration. The National High Magnetic Field Laboratory at Florida State University is a tremendous scientific resource.
The introduction of Part 1 of the two part series:
Linking molecular structure to aggregation potential requires detailed molecular level information. On a bulk scale, asphaltenes are more aromatic and contain more polar compounds than their parent crude oils. However, recent works have started to illuminate the importance of wax-like interactions between more aliphatic compounds that may contribute to asphaltene aggregation. Unstable asphaltenes have also been shown to have higher binding capacities for alkanes and waxes.(7) Berrueco et al. observed a correlation between decreases in fluorescence intensity and UV absorbance in the largest, excluded molecular weight regime of GPC fractions from asphaltenes, petroleum pitch, and coal-derived materials.(8?10) They hypothesized that compounds in the largest, excluded GPC peak may be larger and more aliphatic.(10) Characterization by Fourier transform ion cyclotron resonance mass spectrometry (FT-ICR-MS) for GPC aggregate fractions collected from a typical atmospheric residue revealed a surprisingly strong correlation between nanoaggregation potential and decreased aromaticity.(11) Large, very aliphatic compounds with extremely low ionization efficiencies comprised the largest, most aggregated fractions...
... Trace metals present in crude oils also complicate refinery processes by potentially deactivating hydrotreatment and hydrocracking catalysts. Vanadium, nickel, and iron are typically the most abundant metals found in petroleum products. Structurally, these metals are incorporated into heterocyclic macrocycles with four modified pyrrole subunits, known as porphyrins.(17,18) The forces driving asphaltene aggregation are not well understood: although metal-containing petroporphyrins are greatly enriched in precipitated asphaltenes, the nature of their involvement is unknown.(19) To probe the forces driving asphaltene aggregation in a laboratory, gel permeation chromatography (GPC) acts as a proxy for real-world aggregation. However, it is not entirely clear how well on-column nanoaggregation mimics that of asphaltene aggregation in the field.
Inductively coupled plasma mass spectrometry (ICP-MS) coupled with GPC yields quantitative chromatograms, commonly called size distributions or size profiles, for individual elements. For porphyrinic metals like vanadium and nickel, GPC chromatograms generally yield trimodal/multimodal aggregate size profiles sufficiently unique to act as “fingerprints” for petroleum samples.(20,21)...
Two of the most important analytical tools in chemistry, NMR and mass spectrometry, depend on magnetic fields. The absolute most sensitive mass spectrometers in the world, those with the highest mass resolution, are Fourier Transform Ion Cyclotron Resonance Mass Spectrometers, and a major manufacturer of these is Bruker, which is the company that built the 21 Tesla magnet at the Lab. The use of this magnet in mass spectrometry allows for the most sensitive analysis ever conducted anywhere.
The authors continue:
Aromaticity in chemistry refers to a quantum chemical effect in which a ring system contains (2n+2) "pi" electrons where n is an integer including zero. Aromatic rings are stabilized when compared to non aromatic systems of carbon atoms, the latter being termed "aliphatic" above. (The size of the ring also contributes to aromaticity: An eight membered ring with 2 pi electrons is not aromatic, a three membered ring with two pi electrons is aromatic.) The degree to which a ring system is aromatic can be crudely examined (for very complex systems like asphaltenes) by considering "double bond equivalents" herein called "DBEs."
Besides carbon, asphaltenes also contain quantities of sulfur, nitrogen and oxygen. Under certain circumstances these atoms can donate electrons to a ring system, inducing a degree of aromaticity. For instance, furan, a five membered ring containing an oxygen, derivatives of which has been the subject of considerable attention in connection with biofuels made from non-food biomaterials, has a measurable degree aromaticity.
The asphaltenes were solvated in xylene, aromatic molecules which are a mixture of dimethylbenzenes, and subject to gel permeation chromatography (GPC) a chromatographic technique which separates molecules (somewhat crudely) on the basis of their molecular size, which generally correlates closely with molecular weight. The elution through the chromatograph columns utilized THF, tetrahydrofuran, which is made by hydrogenating furan, mentioned above, or by condensation of a product of the dangerous fossil fuel industry, butadiene. A small portion of the eluted asphaltenes were diverted to a commercial high resolution inductively coupled plasma (ICP) high resolution mass spectrometer designed to measure "heteroatoms," those atoms which are not carbon or hydrogen. These were used to monitor sulfur, using the isotope with a mass of 32, the most common sulfur isotope, presumably in such a way as to break up interfering O2 molecules, vanadium-51, the only stable isotope of this element, (natural vanadium is very slightly radioactive owing to the very rare radioactive isotope vanadium-50), and Nickel-58. It does not seem iron was monitored.
A word on why these metals are important in studying aggregation: Metals are known to complex with certain aromatic rings, in particular cyclopentadiene anions, but also with the molecules described above as porphyrins. The presence of porphyrins is definitely an artifact of the fact that the origin of most dangerous fossil fuels was from biomass; dangerous fossil fuels are stored solar energy. Porphyrins are very common in biological systems, two metal coordinating porphyrins are generally known by the general public. Chlorophyll contains a porphyrin structure coordinating magnesium, and hemoglobulin a porphyrin coordinating iron. (There are many other examples.) The authors remark that the fractions that are highly aggregated often contain metals, and part of their effort is to explore why this is.
Some pictures from the text of Part 1:
Sulfur, Vanadium and Nickel:

The caption:
In general, the heaviest molecules elute first in GCP.
The distribution and ratios of hetero atoms, sulfur, nitrogen and oxygen in the various fractions:

The caption:
The lower the ratio of hydrogen to carbon, the more aromatic character a molecule is likely to have:

The caption:
In general, asphaltenes, especially given their aromatic character, are difficult to ionize. A typical ionization technique - for which a Nobel Prize was awarded, is ESI - electrospray ionization - but in this case, another method, more suitable to the ionization of aromatics, APPI - atmospheric pressure photoionization by which the ionization is achieved by the use of very high energy ultraviolet radiation was utilized, owing the expected aromatic nature of asphaltenes. The ionization efficiency was obtained by recording the number of ions collected as a function of time:

The caption:
It is important to note the different scales on the y axes in the graphic above.
In the next series of graphics, the double bond equivalents within molecules within the fractions are represented. The closer this distribution - these in effect a three dimensional graphics where the third dimension is represented by color - lies to the red line in each graphic, the more highly aromatic these asphaltenes are:

The caption:

The caption:
To some extent, this data is a function of the analytical method.
The authors write:
In this case, the samples were collected by fractionation and analyzed by direct infusion. In part 2, the limitations of this procedure are addressed by the use of in line LC/MS/MS using the 21 Tesla magnet.
The conclusion of part 1:
Part 2 begins thus:
Despite the challenges associated with the analysis of asphaltenes, recent work has begun to reveal that waxlike interactions between more aliphatic compounds may play a more important role in asphaltene aggregation than previously known...
...Gel permeation chromatography (GPC) can help probe the forces driving asphaltene aggregation by acting as a proxy for studying aggregation in a laboratory. GPC is often coupled online with detection by inductively coupled plasma mass spectrometry (ICP MS), thereby enabling the quantitative determination of individual elements. GPC ICP MS chromatograms are commonly termed size distributions or size profiles. Most commonly, sulfur is monitored along with the most abundant heavy metals in petroleum products (vanadium, nickel, and iron). Heavy metals are of interest due to their potential to deactivate hydrotreatment and hydrocracking catalysts during upgrading and refinery processes. Vanadium, nickel, and iron exist structurally in petroleum as porphyrins (heterocyclic macrocycles with four modified pyrrole subunits).(20,21) Metal-containing petroporphyrins are enriched in precipitated asphaltenes, but their exact role in asphaltene aggregation is unknown.(22) GPC ICP MS chromatograms for porphyrinic metals typically exhibit multimodal/trimodal aggregate size distributions that provide “fingerprints” for petroleum samples.(23,24)...
...In the analysis of complex mixtures, especially asphaltenes, ionization biases arise from differences in ionization efficiencies and aggregation tendencies, resulting in the preferential detection of the species that ionize most efficiently. Chromatographic separations help to overcome ionization biases by simplifying the sample matrix,(2,30) but just as important is the choice of ionization method. Positive-ion atmospheric pressure photoionization ((+)APPI) is widely thought to be the most compatible method for asphaltenes.(5,31,32) Despite the well-known ionization biases of aromatic compounds, APPI ionizes more uniformly compared to electrospray, which is why it was selected for this study,(5,33) which is the second installment of a study that investigates the aggregation tendencies and molecular composition of the PetroPhase 2017 asphaltene sample by use of GPC. In part 1, GPC aggregate fractions were collected from the PetroPhase 2017 asphaltene sample and analyzed by direct infusion.(34) Monomer ion yields and aggregation state were strongly correlated. The asphaltene fractions that were most aggregated ionized ?1000 times less efficiently than the least aggregated fractions in the whole crude oil...
And a rationale for the improvement at 21 Tesla:
From the experimental section, the mass resolution that would make any mass spectrometrist weep with envy:
Resolution envy is a terrible vice.
This mass resolution is basically an order of magnitude greater than the very best common commercial instruments.
Some pictures from the text:

The caption:

The caption:
TIC is "total ion current" a measure of the number of ions being recorded in a unit of elution time. The N4 focus is particularly important to represent porphyrins, which are macrocyclic rings with 4 internal rings, each of which contains one nitrogen.
More N4 related stuff:

The caption:
Some data on the presence of sulfur, and an unexpected finding with respect to ?–? stacking:

The caption:
Some more along these lines:

The caption:

The caption:

The caption:
Some commentary from the paper in connection with figure 8:
This suggests that the bigger asphaltenes are not actually graphene like fragments.
Figure 8:

The caption:

The caption:

The caption:
The following is the cartoon from the abstract page that kind of "rubs it in" about the incredible mass resolution observed with this instrument.

The overall conclusion from this two part work:
Sigh...
I have to admit that as much as I hate dangerous fuels and want them banned as quickly as is humanly and humanely possible, I certainly took pleasure in reading this paper about a problem in the dangerous fossil fuel industry, and, in any case, as noted in the turgid and highly esoteric text above, I see asphaltenes as a potential means to sequester carbon dioxide removed from the air via biomass.
It is important to note that this very powerful instrument can do many incredible things other than to address problems in the dangerous fossil fuel industry. The National High Magnetic Field Laboratory can serve to solve many intractable biological problems, in particular those associated with human disease, as well as addressing many severe environmental problems.
In these times of public insanity, it is wonderful to take a break and recognize that great scientific tools still exist and have yet to be wrecked along with our Constitution and our Country.
Have a nice evening.
A Convergent Synthesis Scheme for Overcoming Virginiamycin Antibiotic Resistance.
The paper I'll discuss in this post is this one: Synthetic group A streptogramin antibiotics that overcome Vat resistance (Seipel et al., Nature (2020). https://doi.org/10.1038/s41586-020-2761-3)
Now that we are experiencing a global pandemic, we are more familiar with the state of affairs that existed for most of human history; deadly infectious agents routinely killed people in huge numbers, with the result that life expectancy was close to 1/2 of what it is today. My grandmother for instance, died in her early 40's from a bacterial infection - my mother was 11 years old - that today might have easily been cured with penicillin, or any other of a number of antibiotics in our arsenal.
However, since pathogenic bacteria have short doubling times, with many generations passing in a single day, there is ample opportunity for them to rapidly evolve resistance to those drugs, thus making them ultimately useless.
There is a big economic problem with anti-infectious medications and that is that they cure diseases. This is very different than is the case with, say, a blood pressure medicine that manages but doesn't cure the disease. The innovator company can collect sales for as long as they can keep its patent life going. Our economic system, given the extremely high cost and extremely risky nature of investing in the discovery of new drugs, does not select for curative agents (unless the disease is so widespread that sales will be enormous even if people are cured).
Worse from an economic standpoint is that if a drug can address a resistant strain that has evolved resistance to existing common antibiotics - well trained doctors will not write scrips for it unless all other medications have failed. This is responsible medicine: It follows that a drug that cures diseases that cannot be cured by "ordinary" means, will not sell.
When I was a kid, I briefly managed a combinatorial chemistry lab for a company that claimed - as marketing - that it was making tools that would enable the discovery of fifty drugs per year. The chemical libraries that I and my team made for this effort, under the direction of the chief scientific officer, didn't develop any drugs. Part way along in this effort, I figured out why this was inevitable, but nobody wanted to hear what I had to say, and I quit and found another job. (No, it was not as bad as Theranos; they were not making stuff up. It was just that the scientific assumptions did not hold experimental water.)
The paper I've cited above is kind of "chemical library" - like, a kind of better steered combinatorial chemistry. Don't give up the ship as they say. It is far superior in focus to what my company was doing 30 odd years ago.
From the introduction:
Streptogramin antibiotics comprise two structurally distinct groups (A and B)3 (Extended Data Fig. 1a) that act synergistically to achieve bactericidal activity in many organisms7 by inhibiting the bacterial ribosome8. Group A antibiotics bind to the peptidyl transferase centre (PTC) and increase affinity for the group B component in the adjacent nascent peptide exit tunnel9. Resistance to the A component mediates high-level resistance to the combination, whereas resistance to the B component results in intermediate resistance10. Similar to other antibiotics that target the PTC, resistance to group A streptogramins can be mediated by the ATP-binding cassette F (ABC-F) family proteins that dislodge antibiotics11 or by Cfr methylases that methylate A2503 of the 23S rRNA to sterically block binding12. A specific resistance mechanism for group A streptogramins is deactivation by virginiamycin acetyltransferases (Vats)2. These proteins acetylate the C14 alcohol, resulting in steric interference and disruption of a crucial hydrogen bond. The combination of vat(A) and vgb(A) genes (which deactivate the B component) is the most clinically relevant streptogramin-resistance genotype in S. aureus in France, where streptogramins (under the trade name Pyostacine) are used orally for skin and soft tissue infections13,14 as well as bone and joint infections15. Semisynthesis has improved water solubility (for example, Synercid16) and increased potency (for example, NXL-10317), but methods to overcome resistance to the class have yet to be discovered. Fully synthetic routes to group A streptogramins have been previously developed18,19,20,21,22,23,24,25,26,27,28, but these routes have not been applied to the synthesis of new analogues. Here we report optimization of our initially reported route18 and its application to the synthesis of analogues designed to overcome streptogramin resistance.
Streptogramins are antibiotics whose carbons are arranged (on a molecular scale of course) in large rings, rings much larger than the 5, 6, 7 and 8 member rings that are fairly common in chemistry.
The basic structure is suggested by this combinatorial chemistry synthetic scheme, Figure 1:
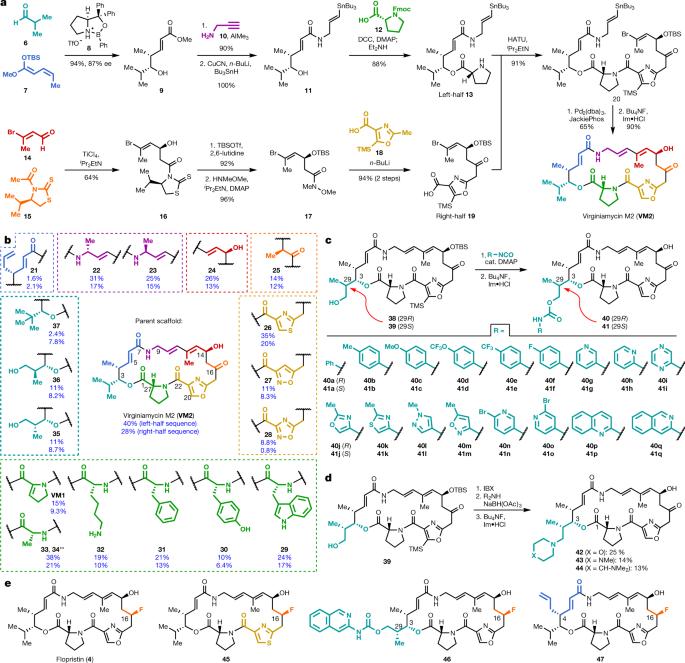
The caption:
In antibiotic talk, "MIC" is called the "minimum inhibitory concentration," the lower the number, the better the antibiotic:

The caption:
Years ago, the testing of potential drugs from chemical libraries basically depended on ligand binding assays. Thing have gotten far more sophisticated and now relies on actual measurement of protein-drug interactions:
To explore the structural basis for antimicrobial activity, we characterized several analogues bound to the E. coli ribosome using cryo-EM (Fig. 3c, d, Extended Data Figs. 5, 6). The PTC is highly conserved across pathogenic species of bacteria, and the E. coli ribosome is an appropriate model for group A streptogramin binding in both Gram-negative and Gram-positive organisms9. The 2.6-Å structure of analogue 47 bound to the ribosome clearly reveals the position of the C4-allyl extension, which projects towards the streptogramin B binding site and makes contacts with A2062, U2585 and U2586 (Extended Data Fig. 5). This extension also adopts a less strained conformation when ribosome-bound than when VatA-bound (calculated ?2.3 kcal mol?1) (Extended Data Fig. 7, Extended Data Table 2). This difference, along with protein conformational changes (Fig. 3b), could contribute to the observed differences in acetylation rates between 4 and 47. In the presence of VS1, the C4 extension adopts a strained conformation similar to its conformation in VatA but is probably stabilized by hydrophobic interactions with the B component (Fig. 3d).
Ligand strain may also have a role in the efficacy of 46. Predicted low-energy conformations of 46 position the arylcarbamate extension directly over the macrocycle (Extended Data Fig. 7); however, the structures of 46 bound to the ribosome in the presence or absence of VS1 (Fig. 3b, Extended Data Fig. 5) showed density for the extension in the P-site. The isoquinoline portion of the extension sits between A2602 and C2452, without making specific contacts with either. The proximity of C29 to U2585 may explain the difference in activity between the two diastereomeric series at this position (40a-q and 41a-q) (Fig. 1c).
A cartoon about the binding:
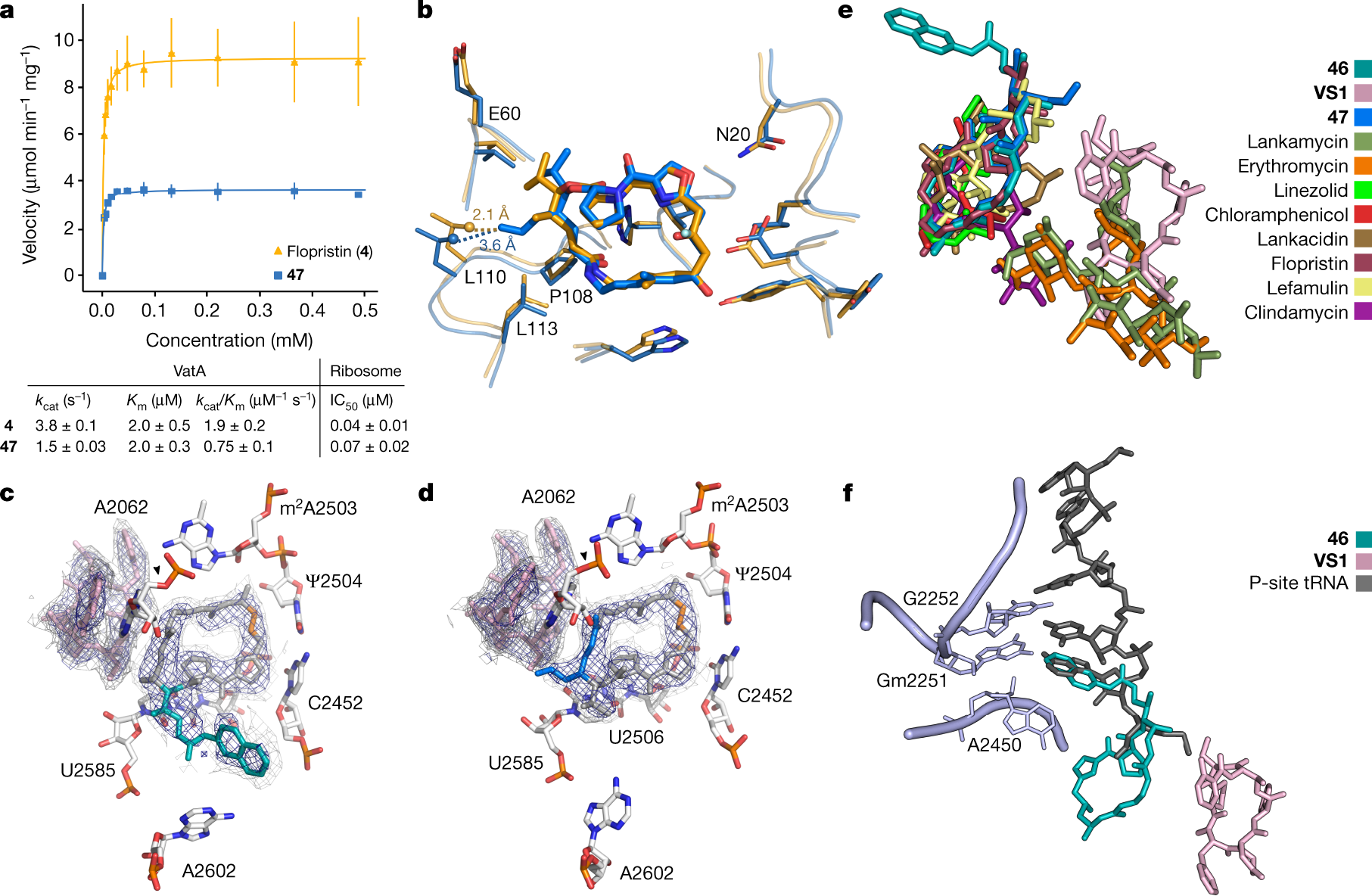
The caption:
Despite all this nonsense in the government, a true Confederacy of Dunces, to steal a line from John Kennedy Toole, scientists are still working to save our pathetic little butts from things other than Covid.
This is a cute little paper (actually a very, very good paper) that evokes a certain sense of nostalgia in me.
Life is interesting as hell, and then you die.
Have a nice day tomorrow.
...til Birnam wood comes to Dunsinane...
Cindy McCain endorsed a Democrat.
All of Shakespeare's tragedies concluded with a bloody justice, the victims honored, the villains destroyed by their own hubris.
"Despair thy charm and let the angel thought still have served tell thee. Macduff was from his mother's womb untimely ripped..."
Untimely ripped. Shakespeare wrote about real life, and although much in the United States States has been lost and cannot be restored i feel very much as if we are in the last act.
My son will not vote in New Jersey.
He registered to vote in Pennsylvania, where he's a student, and where he lives with his girlfriend.
He says Pennsylvania matters more than New Jersey.
His girlfriend - also from New Jersey - did the same.
I don't think, in any case, that he'll be coming home again. He graduates in December, will probably stay there for graduate school, seems to be in love, and there's no chance, absolutely none, that New Jersey will vote for the idiot racist fascist.
Helplessly Hoping
For what it's worth...
Population genomics of the Viking world
The paper I'll discuss in this post is this one: Population genomics of the Viking world (Eske Willerslev et al., Nature volume 585, pages390–396(2020))
According to "23 and me" analysis of my sister's-in-law genome, my wife is 30% (or maybe 32.38712% - I forget) French, which is news to me. I always kind of thought she was an American, and I have spent a lot of time in France among French people, I don't think my wife is French at all, or knows about how to be French, or wants to know about how to be French.
It gets a little silly in my view. These genetic ancestry companies, by the way, don't make any money by telling you about your remote relation to Anne Bolelyn's cousin's wife's brother's grandfather's adopted sister's great uncle's wife's bastard son. They make money by selling genetic distribution data to pharmaceutical companies, so they can figure out which diseases and syndromes are likely to be most profitable to manage and/or (god forbid) cure.
Nevertheless, there probably is some value in population genetics, and one area of active research is in physical anthropology, which can, if interpreted properly, surprise us by showing the commonality of our humanity, along with the beauty of diversity.
I came across this paper yesterday in desultory wandering around the literature, and it caught my eye because...because...well, honestly, I have no idea why it caught my eye but it seems to have done so. Following the references therein helped me make fun of one of my former intellectual conceits - which has now been considered to be vaguely (or maybe even grossly) racist - with respect to the disappearance of the Greenland Norse.
The main author listed in the reference next to the link - the authorship is quite a crowd - Eske Willerslev, is a pioneer in population genetics based anthropology, and according to his web page, is a world famous scientist and adventurer. Until yesterday, I never heard of him, but I'm a somewhat limited human being. For example, I know next to nothing about the Kardashian family except that one of them is apparently married to a famous African American Trumper, although I have no idea why the Trumper in question is famous.
Anyway, the abstract is available at the link above. From the introduction:
To explore the genomic history of the Viking Age, we shotgun-sequenced DNA extracted from 442 human remains from archaeological sites dating from the Bronze Age (about 2400 BC) to the Early Modern period (about AD 1600) (Fig. 1, Extended Data Fig. 1). The data from these ancient individuals were analysed together with published data from 3,855 present-day individuals across two reference panels (Supplementary Note 6), and data from 1,118 ancient individuals (Supplementary Table 3).
Here is a picture of some of the participants in this study, none of whom apparently gave "informed consent" to participate in the study, although there seems to have been no complaints from these subjects or their families about their uninformed lack of consent, which is probably a little less of ethical concern than say, the case of Henrietta Lacks, because the case of Henrietta Lacks involves some very questionable historical sociological implications:

The caption:
Examples of a few archaeological Viking Age sites and samples used in this study. a, Salme II ship burial site of the Early Viking Age, excavated in present-day Estonia: schematic of skeletons (top left) and aerial images of skeletons (top right, and bottom). b, Ridgeway Hill mass grave dated to the tenth or eleventh century AD, located on the crest of Ridgeway Hill near Weymouth, on the south coast of England (reproduced with permission from Dorset County Council/Oxford Archaeology). Around 50 predominantly young adult male individuals were excavated. c, The site of Balladoole, around AD 900, a Viking was buried in an oak ship at Balladoole (Arbory) in the south east of the Isle of Man. d, Viking Age archaeological site in Varnhem, in Skara municipality (Sweden). Schematic map of the church foundation (left) and the excavated graves (red markings) at the early Christian cemetery in Varnhem; foundations of the Viking Age stone church in Varnhem (middle) and the remains of a 182-cm-long male individual (no. 17) buried in a lime stone coffin close to the church foundations (right).
By the way, the supplementary files, which are probably open sourced, have fascinating details about all the dead people participating in the study. The files are 178 pages long, if you have some time to kill, and are found here: Supplementary information
The text continues:
The Viking Age Scandinavian individuals of our study fall broadly within the diversity of ancient European individuals from the Bronze Age and later (Fig. 2, Extended Data Figs. 2, 3, Supplementary Note 8), but with subtle differences among the groups that indicate complex fine-scale structure. For example, many Viking Age individuals from the island of Gotland cluster with Bronze Age individuals from the Baltic region, which indicates mobility across the Baltic Sea (Fig. 2, Extended Data Fig. 3). Using f4-statistics to contrast genetic affinities with steppe pastoralists and Neolithic farmers, we find that Viking Age individuals from Norway are distributed in a manner similar to that of earlier Iron Age individuals, whereas many Viking Age individuals from Sweden and Denmark show a greater affinity to Neolithic farmers from Anatolia (Extended Data Fig. 4a). Using the qpAdm program, we find that the majority of groups can be modelled as three-way mixtures of hunter-gatherer, farmer and steppe-related ancestry. The three-way model was rejected for some groups from Sweden, Norway and the Baltic region, which could be fit using four-way models that additionally included either Caucasus hunter-gatherer or East-Asian-related ancestry (Extended Data Figs. 4b, c)—the latter of which is consistent with previously documented gene flow from Siberia5,6,7.
Anatolia is the peninsula now wholly contained by the nation of Turkey, which is currently ruled by a Trump type, apparently. Irrespective of that fact, it's somewhat surprising to find that some Vikings were Turks.
The Viking World according to the authors, Fig. 1: Overview of the Viking Age genomic dataset:

The caption:
The next graphic apparently involves mapping from one real coordinate space to another (2 dimensional) coordinate space as a means of to determine "similarity" graphically. In one case, it's called "MDS" which is an abbreviation for multi-dimensional scaling and another, it's a "UMAP." I was frankly unfamiliar with these terms, which involve some nice mathematics involved in the area of statistics. Most of the statistics I know comes from taking courses in analytical chemistry, and some from osmosis over the years; but I've never actually taken a formal course in statistics, and certainly not in statistical similarity determinations in genomics. Poking around the internet showed me that apparently a UMAP is better than a tSNE, but I'm going in only so far as to say I've sort of, more or less, well, "in a fashion" have been there...
Anyway, similarity mappings, Fig. 2: Genetic structure of Viking Age samples:

The caption:
The authors continue:
The living and the dead, Fig. 3: Genetic structure and diversity of ancient samples.:


The caption:
And finally - trust me, Anne Boleyn is in here somewhere - Fig. 4: Spatiotemporal patterns of Viking and non-Viking ancestry in Europe during the Iron Age, Early Viking Age and Viking Age:



The caption:
Well then...
The authors discuss the disappearance of the Norse settlements in Greenland, an area of the world that is now famously melting at some threat to all of humanity, at least coastal humanity:
From around AD 980 to 1440, southwest Greenland was settled by people of Scandinavian ancestry (probably from Iceland)28,29. The fate of these populations in Greenland remains debated, but probable causes of their disappearance are social or economic processes in Europe (for example, political relations within Scandinavia and changed trading systems) and natural processes, including climatic change29,30,31.
According to our data, the Greenland Norse populations were an admixture between Scandinavians (mostly from Norway) and individuals from the British Isles, similar to the first settlers of Iceland18. We see no evidence of long-term inbreeding in the genomes of Greenlandic Norse individuals, although we have only one high-coverage genome from the later period of occupation of the island (Supplementary Note 10, Supplementary Figs. 10.2, 10.3). This result could favour a relatively brief depopulation scenario, consistent with previous demographic models32 and archaeological findings. We also find no evidence of ancestry from other populations (Palaeo-Eskimo, Inuit or Native American) in the Greenlandic Norse genomes (Supplementary Fig. 9.4)
I always like to wonder through the references in interesting papers, and as such, I found my way to Reference 30, which is this one:
Cultural adaptation, compounding vulnerabilities and conjunctures in Norse Greenland (Dugmore et al., PNAS March 6, 2012 109 (10) 3658-3663)
At the end of the last century, and the beginning of this one, it was intellectually fashionable to embrace the ideas of Jared Diamond, who won the Pulitzer Prize for his book "Guns, Germs and Steel" which was followed by "Collapse: How Societies Choose to Fail or Succeed. I waxed romantic about the latter book, which I read, in a post over at DailyKos - where I was happily banned for reporting a scientific truth, albeit in a very crude way - in order to support the obsessive viewpoint, which is either amusing or dire, of which anyone familiar with my writings will be aware. A large section of the book is about the disappearance of the Greenland Norse, which Diamond attributes to a refusal to eat Salmon because of superstition.
The more recent criticism of Diamond is that his work is overwhelmingly Eurocentric, and quite possibly, racist, at least in a vague, or perhaps obvious, way.
Reference 30 in the paper under discussion in turn contains reference 34, which is to Diamond's "Collapse" about which Dugmore and his coauthors say the following:
I love that locution referring to a logical fallacy, "special pleading," by which the authors dismiss Diamond's contention in "Collapse."
It has a certain trenchant eloquence, don't you think?
If there is any silver lining on the cloud of the near destruction of the United States by agency of having an ignorant racist lead it, it is that old white guys like me have had to reflect on their thinking and their assumptions, to question their immunity from being more than a little racist themselves, irrespective of their previous habits of thinking about themselves. This, I think, is a good thing. One is not really alive without questioning oneself.
The paper that is the general topic of this post concludes like this:
... Some Viking Age Scandinavian locations are relatively homogeneous—particularly mid-Norway, Jutland and the Atlantic settlements. This contrasts with the strong genetic variation of populous coastal and southern trading communities such as in the islands of Gotland and Öland47,48,49. The high genetic heterogeneity in coastal communities implies increased population size...
...Finally, our findings show that Vikings were not simply a direct continuation of Scandinavian Iron Age groups. Instead, we observe gene flow from the south and east into Scandinavia, starting in the Iron Age and continuing throughout the duration of the Viking Age, from an increasing number of sources. Many Viking Age individuals—both within and outside Scandinavia—have high levels of non-Scandinavian ancestry, which suggests ongoing gene flow across Europe.
Perhaps, just perhaps, the "Scandinavians" are more human than Scandinavian.
I trust you are having a pleasant, safe, and healthy Sunday afternoon.
Have you ever woke up in the morning and contemplated the life of Niels Ryberg Finsen?
Me neither.
Niels Ryberg Finsen
If you worry though, that this might happen to you, I advise you not to read the paper in this week's issue of Nature, on the exploration genetic signatures of the Vikings, Population genomics of the Viking world.
You might end up thinking about Niels Ryberg Finsen, and I'm sure you have better things to do.
I probably did too, but this is how my day ended up, finding out who Niels Ryberg Finsen was, and I'm worried that tomorrow might begin in a similar way, contemplating something or another about the Faroe Islands. (I haven't eaten a sheep since I was a child.))
Life is fun and then you die.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512