NNadir
NNadir's JournalI just chatted with customer service at Delta airlines to...
...assure them that given their support for over suppression in their corporate statement, as a one time (precovid) and perhaps future business traveler I will be avoiding their airline like the plague.
Catalyst-free ceramic-carbonate dual phase membrane reactor for the water gas Rxn CO2 separation.
The paper I will discuss in this post is this one: Catalyst-free ceramic-carbonate dual phase membrane reactor for hydrogen production from gasifier syngas (Xueliang Dong, Y.S. Lin, Journal of Membrane Science, Volume 520, 2016, Pages 907-913.)
I was motivated to call this paper up when reading another paper (by the same research group) offering some mathematical modeling of this very interesting device. That paper is this one, published a few weeks ago: Catalyst-Free Ceramic–Carbonate Dual-Phase Membrane Reactors for High-Temperature Water Gas Shift: A Simulation Study (Lie Meng, Oscar Ovalle-Encinia, and Jerry Y. S. Lin, Industrial & Engineering Chemistry Research 2021 60 (9), 3581-3588) This group is out of Arizona State University.
The importance of the water gas shift reaction - it is the source of most of the world's hydrogen, which is overwhelmingly produced by the reformation of dangerous natural gas - cannot be underestimated. Among other things, the world's food supply depends on this reaction, as it is widely used to make ammonia.
I oppose the use of all dangerous fossil fuels, and as such, I'm always imagining all kinds of Rube Goldberg heat networks to make carbon monoxide without the use of dangerous natural gas, and it occurs to me that this magnificent biphasic membrane is the sort of thing that could be plugged into such a scheme.
The second paper cited, the mathematical modeling paper gives a nice overview of the water gas shift reaction (WGS) so I'll excerpt that first, although it focuses on carbon capture and power generation, and not hydrogen for synthetic purposes:
As demonstrated via experiments and simulations, palladium-based metal membrane reactors exhibit extremely high selectivity for hydrogen separation. Augustine et al.(3) developed a palladium membrane reactor for high-temperature WGS at a feed pressure up to 15 bar and achieved a maximum CO conversion of 98.2% with a H2 recovery of 81.2% at 450 °C. However, the palladium membrane suffers from several drawbacks that significantly limit its practicality: hydrogen embrittlement, surface poisoning, and high membrane cost.(4) Modified MFI-type zeolite membranes promise high-temperature-selective hydrogen separation due to good thermal and chemical stability and high hydrogen permeance. However, MFI-type zeolite membranes have relatively low H2 perm-selectivity, which should be improved for use in membrane reactors for WGS.
Many efforts have been devoted to improving the H2/CO2 selectivity of MFI zeolite membranes (pore diameter: 0.55 nm, Knudsen selectivity for H2/CO2: ?4.7), including narrowing the zeolitic pore by catalytic cracking deposition of monosilica.(5) For precise control of pore modification, Wang et al.(6) developed a porous ?-alumina-supported bilayer MFI zeolite membrane consisting of a ZSM-5 layer on a silicalite-1 layer to reduce the aluminum content in the zeolite top layer. At 500 °C, the modified MFI zeolite membrane exhibited a H2 permeance of ?1.2 × 10–7 mol m–2 s–1 Pa–1 and a H2/CO2 selectivity of ?23 in a 24 day long-term test. WGS conducted at temperatures lower than 500 °C is thermodynamically favored to a high CO conversion because of its exothermic nature, indicating, for high-temperature WGS, that the increase in reaction temperature has a negative effect on the equilibrium CO conversion. However, for these H2-selective membrane reactors, the H2 permeance through the membrane drops with decreasing temperature, resulting in a weak shift in WGS and a low H2 recovery rate. Moreover, most modified MFI-type zeolite membranes show a H2/CO2 selectivity below 30, suggesting that a low CO2 capture ratio could be expected.
Another strategy to enhance WGS in the membrane reactor is the use of CO2-permeable membranes. This will avoid or reduce a load of CO2 separation by amine absorption or adsorption process, reducing the costs for carbon capture in the IGCC process. Recently, we developed several high-performance ceramic–carbonate dual-phase (CCDP) membranes, which are high-temperature CO2 separation materials with remarkable CO2 permeance and theoretically infinite CO2 selectivity.
My whole adult life I've been thinking about this in terms of palladium membranes, and so I find this very cool.
The text of the first paper cited is similar to that excerpted above from the second:
As demonstrated via experiments and simulations, palladium-based metal membrane reactors exhibit extremely high selectivity for hydrogen separation. Augustine et al.(3) developed a palladium membrane reactor for high-temperature WGS at a feed pressure up to 15 bar and achieved a maximum CO conversion of 98.2% with a H2 recovery of 81.2% at 450 °C. However, the palladium membrane suffers from several drawbacks that significantly limit its practicality: hydrogen embrittlement, surface poisoning, and high membrane cost.(4) Modified MFI-type zeolite membranes promise high-temperature-selective hydrogen separation due to good thermal and chemical stability and high hydrogen permeance. However, MFI-type zeolite membranes have relatively low H2 perm-selectivity, which should be improved for use in membrane reactors for WGS.
Many efforts have been devoted to improving the H2/CO2 selectivity of MFI zeolite membranes (pore diameter: 0.55 nm, Knudsen selectivity for H2/CO2: ?4.7), including narrowing the zeolitic pore by catalytic cracking deposition of monosilica.(5) For precise control of pore modification, Wang et al.(6) developed a porous ?-alumina-supported bilayer MFI zeolite membrane consisting of a ZSM-5 layer on a silicalite-1 layer to reduce the aluminum content in the zeolite top layer. At 500 °C, the modified MFI zeolite membrane exhibited a H2 permeance of ?1.2 × 10–7 mol m–2 s–1 Pa–1 and a H2/CO2 selectivity of ?23 in a 24 day long-term test. WGS conducted at temperatures lower than 500 °C is thermodynamically favored to a high CO conversion because of its exothermic nature, indicating, for high-temperature WGS, that the increase in reaction temperature has a negative effect on the equilibrium CO conversion. However, for these H2-selective membrane reactors, the H2 permeance through the membrane drops with decreasing temperature, resulting in a weak shift in WGS and a low H2 recovery rate. Moreover, most modified MFI-type zeolite membranes show a H2/CO2 selectivity below 30, suggesting that a low CO2 capture ratio could be expected.
Another strategy to enhance WGS in the membrane reactor is the use of CO2-permeable membranes. This will avoid or reduce a load of CO2 separation by amine absorption or adsorption process, reducing the costs for carbon capture in the IGCC process. Recently, we developed several high-performance ceramic–carbonate dual-phase (CCDP) membranes, which are high-temperature CO2 separation materials with remarkable CO2 permeance and theoretically infinite CO2 selectivity...
This groups molten carbonate membranes in many cases were supported on a type of zeolite known as "MFI zeolite," a type of porous silicate. Here in this paper, the support is a ceramic, "SDC," samarium doped ceria.
Some pictures from the text:

The caption:

The caption:

The caption:
Both papers offer a considerable amount of more detail and analysis.
From the conclusion of the paper:
To me, IGCC is an appalling idea, but the application of this technology may be very useful elsewhere. There is a heat engine cycle known as the "Allam Cycle" which with (unlike the cycles original conception) an external heat source, can be utilized to produce carbon monoxide from waste carbon materials, plastics, other garbage, biomass. (This is called "dry reforming.) Incorporating this very interesting technology suggests some very interesting possibilities.
I'm having a nice week, a molten carbonate kind of week: Electrolysis of Lithium-Free Molten Carbonates.
If you were to tell me that you found getting excited about molten carbonates is well, "a little off," well it's OK. To each his or her own.
Have a nice day tomorrow.
Don't worry, be happy. We can make ethylene glycol with solar and wind power from, um, coal.
I'm not really even going to discuss this paper other than to say you can make anything "green" simply by drawing a picture of a wind turbine and a solar cell. The paper is this one: Low-Carbon Path of Geographically Matched Hybrid Energy Utilization: A Coal-To-Ethylene Glycol Process with Hydrogen from the Coupled Wind/Solar Power
(Xiaofei Shi, Qi Wang, Siyu Yang, and Yu Qian ACS Sustainable Chemistry & Engineering 2021 9 (12), 4583-4599)
Here's the graphic at the abstract:

Don't you just feel all "green," just looking at it?
Don't worry, be happy.
Excerpt from the opening of the paper:
Currently, there are two routes of large-scale EG production in China: the oil-based ethylene oxide hydration route (OtEG) and the coal-based oxalate hydrogenation route [coal-to-EG (CtEG)].(4,5) As there are not enough oil reserves, CtEG is of great interest to China. As of September 2020, there have been more than 30 CtEG plants in operation in China, with a total annual EG production capacity of more than 10 million tons. In addition, there are more than 20 projects under construction or in planning. It is estimated that nearly 50% of EG production in China will be in the catalog of coal-based routes in 2025.(6)
The hydrogen-to-carbon mole ratio of coal is generally less than 1.0, which is far less than that of the EG product in the range of 1.95–2.05. Water gas shift (WGS) is used to convert H2O and CO into CO2 and H2, resulting in carbon emissions up to 3.1 t/t-EG.(7) Therefore, reduction of CO2 emissions in the process is a critical and technical challenge. Integration of hydrogen-rich resources with a coal-based process is considered an effective way to reduce carbon emissions. A number of scholars have coupled coke oven gas and coal chemical processes to produce olefins, natural gas, methanol, and EG.(8?11) Compared with the traditional coal-based process, these integrated systems improve the exergy efficiency and carbon efficiency in some way, with a reasonable economic performance. From the perspective of life cycle assessment, coke, as the other product of the coke oven gas production process, will cause high-intensity carbon emissions during the combustion process.(12,13)
Looking for a true low-carbon hydrogen source is of great significance to the clean utilization of coal. Increasing global energy demand and attention to sustainable development have promoted the development of wind and solar energy utilization technologies.(14) Wind energy and solar energy are widely distributed and global-wide available. The global potentials of wind and solar power generation far exceed the current total energy consumption.(15) As of 2019, the global installed capacity of wind power reached 1270.0 TW h, and the installed capacity of solar power reached 584.6 TW h. In the past 10 years, the proportion of wind power and solar power generation in the total global power generation increased from 3.0% to 9.3%.(16) With the rapid increase of wind power and solar power capacity, a large amount of unstable electricity puts a greater pressure on the grid, making power curtailment a common phenomenon.(17)
Hydrogen production from electrolysis of water is a mature technology that has been applied on a large scale.(18) The route of electrolytic hydrogen production using wind power and solar power is one of the most environmentally friendly methods.(19)
We're just all awash in that wind and solar hydrogen, aren't we? I remember hearing here, some ten or fifteen years ago, all about that great wind to hydrogen pilot plant on the Norwegian Island of Utsira, and how it was going to change the world.
Meanwhile, at the Mauna Loa Carbon Dioxide Observatory, this week:
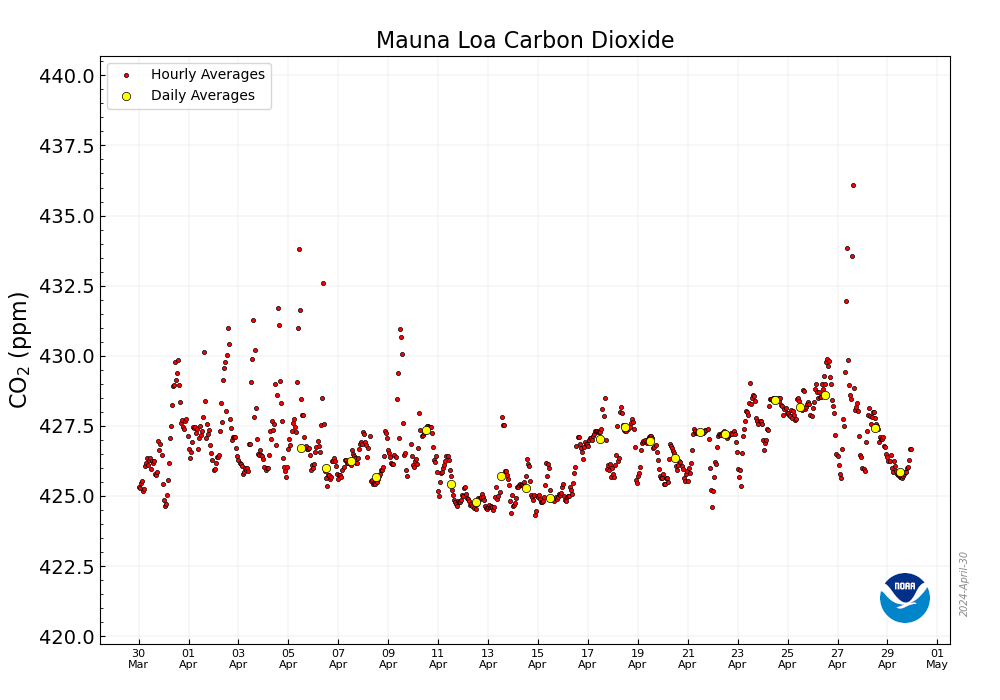
Less than 10 years ago, just after or around the time I was hearing all about the wonderful wind to hydrogen plant on the Norwegian Island of Utsira - it briefly powered 10 homes before being shut off for "lessons learned" - we passed 400 ppm for the first time. That was in 2014.
Don't worry be happy.
Schematic of the wind and solar and coal to ethylene glycol plant:

The caption:
I assume it stands for "Wind Power Coal to Ethylene Glycol.
I was a child of the 1970's, and frankly, my generation got polyester between our ears.
History will not forgive us; nor should it.
Don't worry; be happy.
Have a nice day tomorrow.
Calix[4]trap: A Bioinspired Host Equipped with Dual Selection Mechanisms
The paper I'll discuss in this post is this one: Calix (4) trap A Bioinspired Host Equipped with Dual Selection Mechanisms (Zhenchuang Xu, Nie Fang, and Yanchuan Zhao, Journal of the American Chemical Society 2021 143 (8), 3162-3168)
(The HTML codes here did not allow for me to write the title in the link as written in the actual title of the paper: Calix[4]trap: A Bioinspired Host Equipped with Dual Selection Mechanisms)
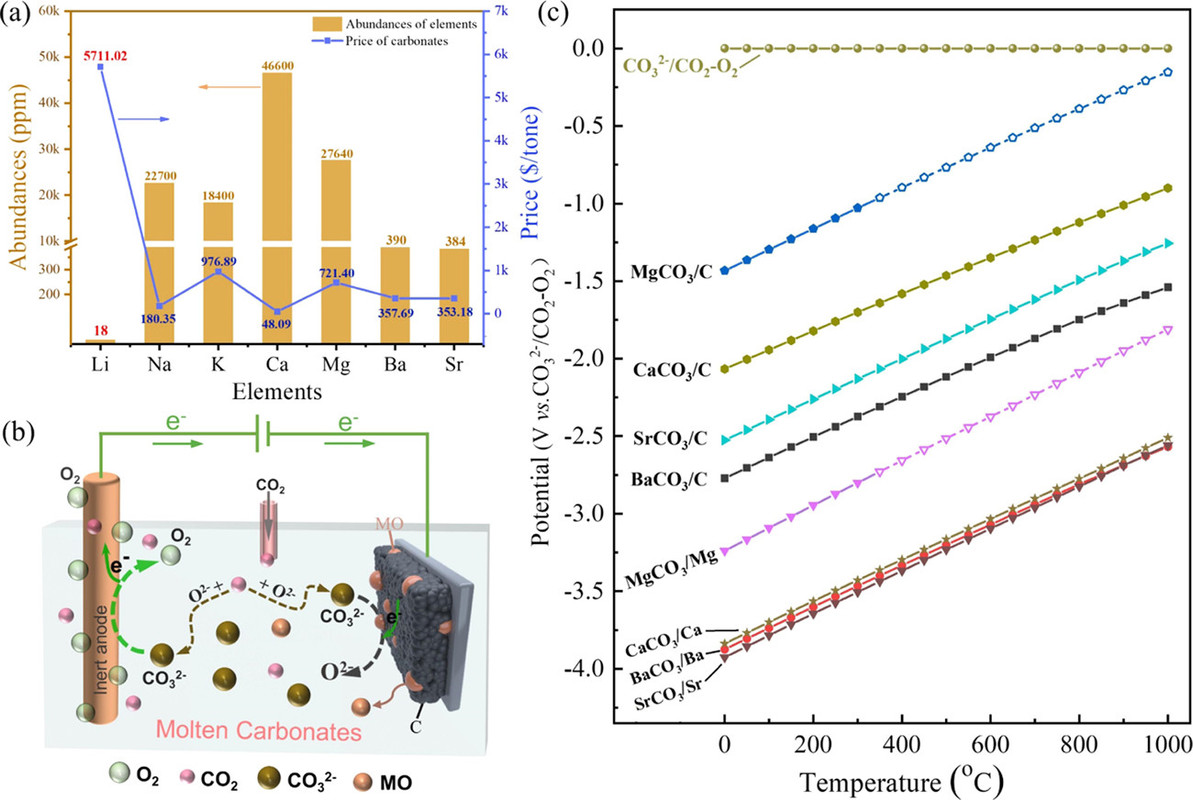
I am always interested in the separation of group I and group II elements in the periodic table. Group I consists of the elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb) and cesium (Cs). (Let's forget about francium, a laboratory curiosity.) Group II consists of the elements beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba) and radium (Ra).
These separations, particularly for the heavier members, play an important role, among many other places, in the recovery of important valuable materials from used nuclear fuels, and in the clean up of historical sites contaminated with fission products.
A post I wrote yesterday in this space inspired some thinking about group II separations (although it was actually about the electrochemically driven "combustion in reverse," the reduction of carbon dioxide to elemental carbon). It seems to me that this particular technology is a path to addressing some problematic environmental problems connected with the production of aluminum metal, including, but not limited to carbon emissions from the "green" anodes.
Today I stumbled on the paper cited at the outset of this post, and thought I'd just point out some very wonderful scientific thinking.
From the introduction to the paper:
A little while later they take on the task of answering their scientific question.
Inspired by the unique structure and properties of valinomycin and K+ channels, we sought to create a biomimetic ion host that discriminates ions through both thermodynamic and kinetic selection mechanisms. Given the fact many ion hosts display excellent thermodynamic ion selectivity,(22,23) our effort is directed to confer established ion hosts with a tunnel-like ion transport path that is essential for kinetically varied ion binding. We see the 1,3-alternate calix[4]arenecrown-5 (1) as a great candidate on which to perform the structural reengineering because it functionally resembles valinomycin and displays recording-setting K+/Na+ selectivity.(24,25) Unlike the orderly ion transport occurring within ion channels, metal ions approach 1’s central cavity from diverse directions, resulting in rapid and poorly controlled binding processes. Despite the exceptional K+/Na+ selectivity, 1 fails to effectively discriminate K+ from metal ions of comparable sizes, such as Rb+ and Ba2+. We thus wonder whether the not-yet-owned discrimination ability could be conferred by a confined binding tunnel biomimetic to K+ channel, where ions are uptaken with distinct rates. To test the feasibility of this idea, we first attempted to encapsulate 1’s ligating etheric oxygens within a confined tunnel to block the original ion binding path.
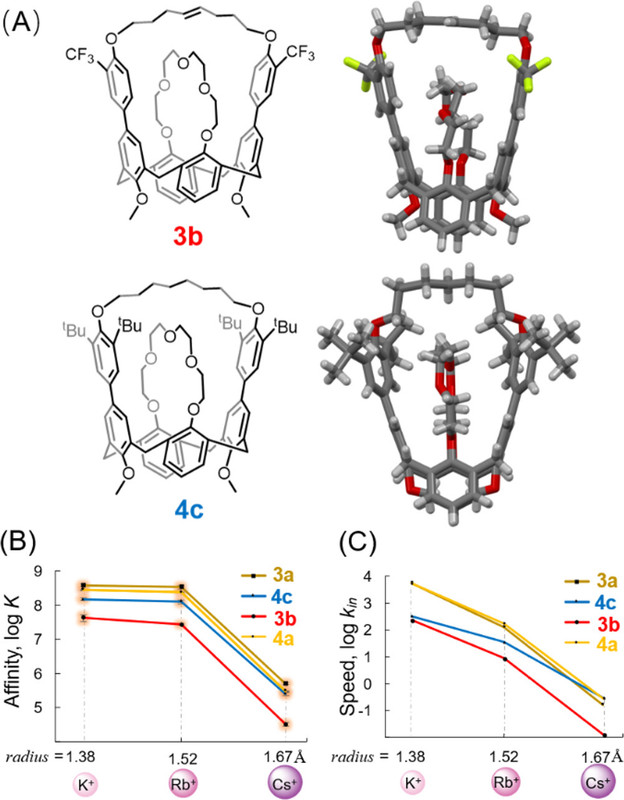
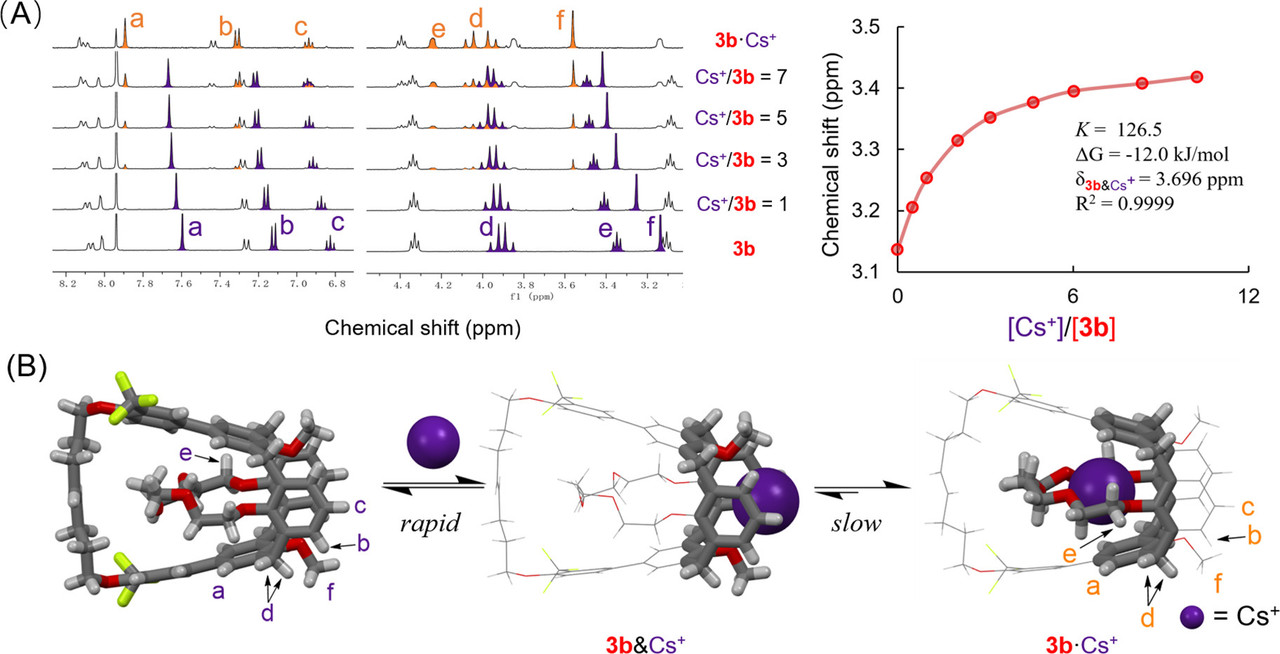
A few pictures from the paper show the experimental path and the results:
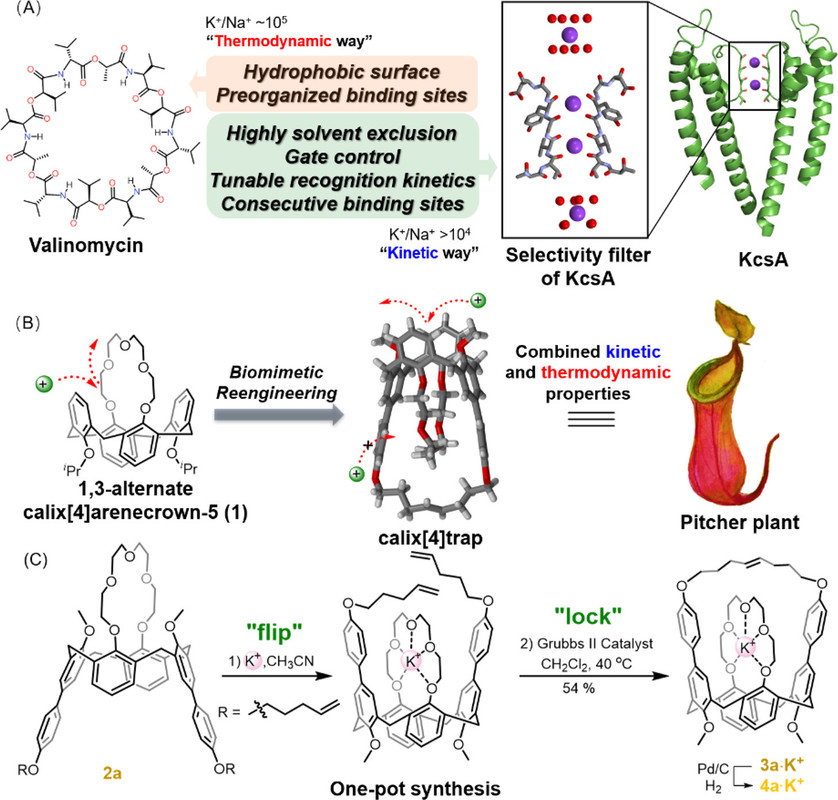
The caption:

The caption:
The caption:
The caption:
From the conclusion to the paper:
It is very unlikely that these interesting reagents will prove to be of industrial importance, other than perhaps inspiring nanostructural templating, nor are they likely to be radiation stable, but this said, they are cool nonetheless.
People of course, find different things inspiring. Musicians and composers and musicologists will find Benjamin Britten's Requiem on a deeper level to which I could ever aspire, for example, and literary types can find deeper meanings in John Ashbery's "Self Portrait in a Convex Mirror" than I could ever dream of understanding.
But as a chemist, I find this paper beautiful, very beautiful.
Electrolysis of Lithium-Free Molten Carbonates
The paper I'll discuss in this post is this one: Electrolysis of Lithium-Free Molten Carbonates (Xiang Chen, Zhuqing Zhao, Jiakang Qu, Beilei Zhang, Xueyong Ding, Yunfeng Geng, Hongwei Xie, Dihua Wang, and Huayi Yin ACS Sustainable Chemistry & Engineering 2021 9 (11), 4167-4174)
I've argued before in this space that electricity is a thermodynamically degraded form of energy: Synthesizing Clean Transportation Fuels from CO2 Will at Least Quintuple the Demand for Electricity.
I have also argued, sometimes while addressing the stupid but oft discussed solar thermal energy fantasy, that the thermochemical splitting of carbon dioxide into carbon monoxide - from which pretty much any industrially produced carbon compound can be made (just add water) - and oxygen is the most efficient approach to carbon dioxide reduction: Cerium Requirements to Split One Billion Tons of Carbon Dioxide, the Nuclear v Solar Thermal cases.
Although for certain reasons I am very fond of the cerium based thermochemical splitting of carbon dioxide, many other such catalytic systems are known for this highly endothermic reaction.
The international symbol for dangerous fossil fuel free energy has become a wind turbine or solar cell graphic object, which is frankly as silly as waving a graphic of a Roman executioner's cross as a symbol for the way to address immorality; both are faith based. It has been experimentally verified, at a cost of trillions of dollars, that wind turbines and solar cells are not even remotely capable of addressing climate change. In fact, the worship of them - and let's be clear that it's nothing more than faith based worship - has led to the acceleration of climate change. The average concentration of the dangerous fossil fuel waste carbon dioxide in the atmosphere measured at the Mauna Loa Carbon Dioxide Observatory during the week beginning on March 19, 2000 was 370.98 ppm. This morning, the latest figure was 417.67 ppm. The current 12 month running average of weekly measured increases over carbon dioxide increases over the last ten years is 24.24 ppm, 2.42 ppm/year. The same 12 moth running average for the week beginning March 19, 2000 was 15.14 ppm, 1.51 ppm/year.
These are facts. Facts matter.
Heckuva job humanity at addressing climate change with all those wind turbines, solar cells and electric cars, heckuva job.
The graphic attached to the introduction of this paper is nonetheless the equivalent of the Roman execution's cross as applied to so called "green" energy. Here it is:

What I like about this paper is that it is cognizant of the fact that matter, specifically the individual elements in the periodic table, is not "renewable." The claim that we can save the world with batteries is no different than the claim that supplies of dangerous petroleum, dangerous natural gas, and dangerous coal are infinite, as well as the claim that places to put the waste, chiefly, but hardly limited to, carbon dioxide is unlimited.
They start out talking about lithium and then consider elements that are far more available, one of which, strontium, catches my eye. Overall this discussion of batteries clearly has some relevance to the "batteries will save us" fantasy.
From the introduction to the paper:
They have a nice graphic on the point:
The caption:
They continue:
The use of molten calcium salts is the essence of the FFC Cambridge process for the electrochemical reduction of metals, which I personally believe should be world changing, even if it is true that electricity is a thermodynamically degraded form of energy.
In saying this, they do raise some issues to be addressed:
These factors are what they examine in the paper, and then propose an electrode to reduce carbon dioxide to carbon, in effect reversing the combustion of the dangerous fossil fuel coal.
This process, which requires the input of a thermodynamically degraded form of energy, electricity, as well as heat suggests an important point that is often over looked, which is this: In order to capture carbon dioxide in air one needs to put more energy into the system than all of the original energy ever released by the original combustion of the dangerous fossil fuel produced. This reality should sober up all the drunken handwaving and wishful thinking that goes on when energy and the environment is discussed, but I confidently predict, on the basis of being a tired old man, that it won't.
Their important concern is thermodynamics:
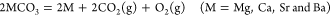 (1)
(1)
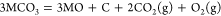 (2)
(2)
If the MO in eq 2 is soluble, then the carbonization reaction between MO and CO2 spontaneously occurs and forms fresh carbonate to maintain the concentration of CO32– of the electrolyte:
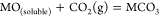 (3)
(3)
The overall reaction of eqs 2 and 3 is
 (4)
(4)
Thermodynamically, alkaline-earth metal carbonates (MCO3, M = Mg, Ca, Sr, and Ba) can be electrochemically converted to carbon at the potential prior to the deposition of the alkaline-earth metal (Figure 1c). Therefore, the deposition of carbon is thermodynamically easier than that of metal. Moreover, the thermodynamic favorability of generating carbon is different depending on the different alkaline-earth metal cations. For example, the thermodynamic deposition potential sequence follows CaCO3 < SrCO3 < BaCO3. In other words, CaCO3 can be reduced to carbon at the potential more positive than that of SrCO3 and BaCO3. Note that the potentials of carbon generation in Na2CO3 and K2CO3 are more negative than that of the deposition of the corresponding alkali metals. Thus, Na2CO3 and K2CO3 are usually employed as the supporting electrolyte.(39,44) Therefore, alkaline-earth carbonates are the solute to be reduced for the carbon generation in MCO3–Na2CO3–K2CO3 (M = Mg, Ca, Sr, and Ba).
The reduction behaviors at a Mo electrode in a variety of carbonates containing different alkaline-earth metals are studied. As shown in Figure 2a, no reduction peaks were observed before the cathodic limit in the pure molten Na2CO3–K2CO3, demonstrating that Mo is an inert material that does not involve in any electrochemical reactions in the selective potential range.
The preliminary experiments investigated the cyclic voltammograms of molten carbonate systems using molybdenum electrodes:
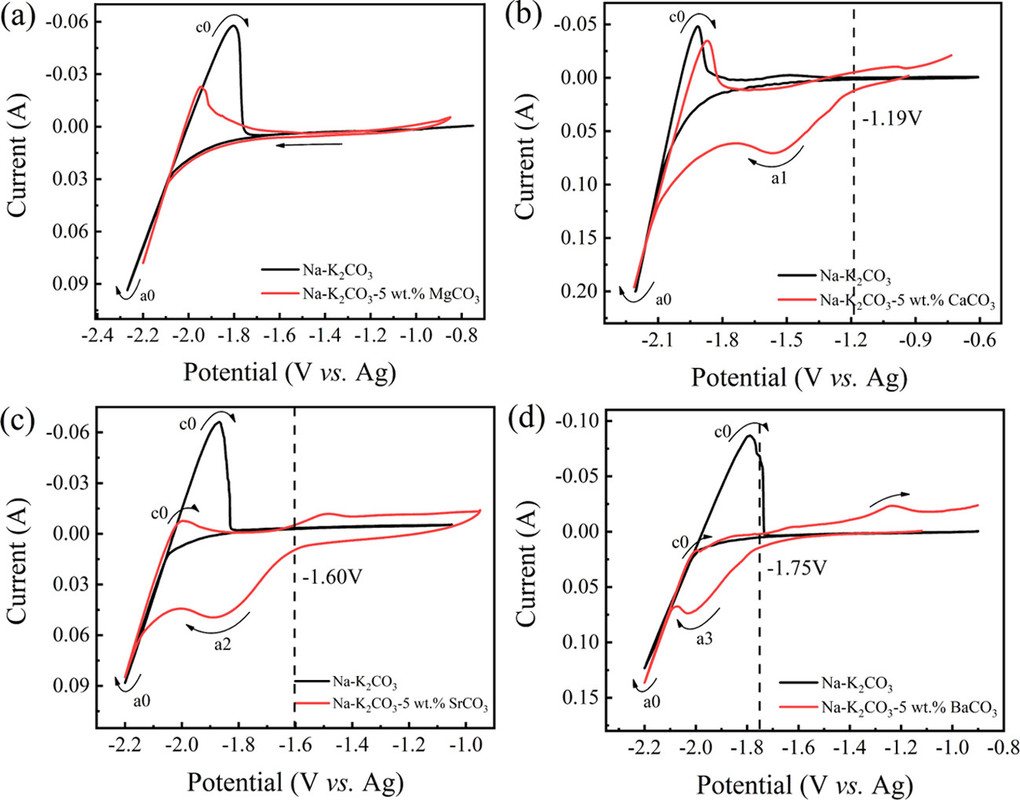
The caption:
Carbon was indeed deposited under these conditions, at a temperature of 750°C in sodium/potassium carbonate melts containing 10% (by weight, surprisingly) of three alkali metal carbonates, those of calcium (as in the FFC Cambridge Process), strontium and barium.
Micrographs of the carbons formed are shown:
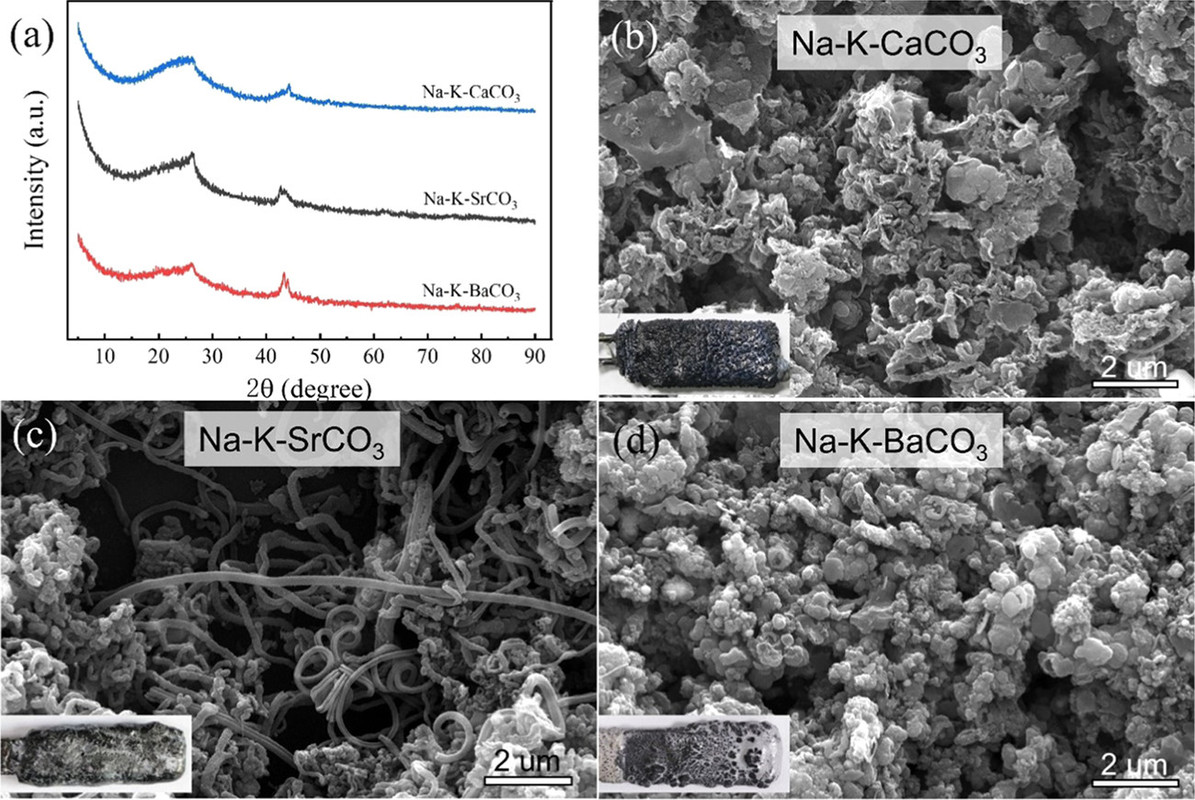
The caption:
The question next turned to finding a stable electrode for the oxidation side of the reaction:
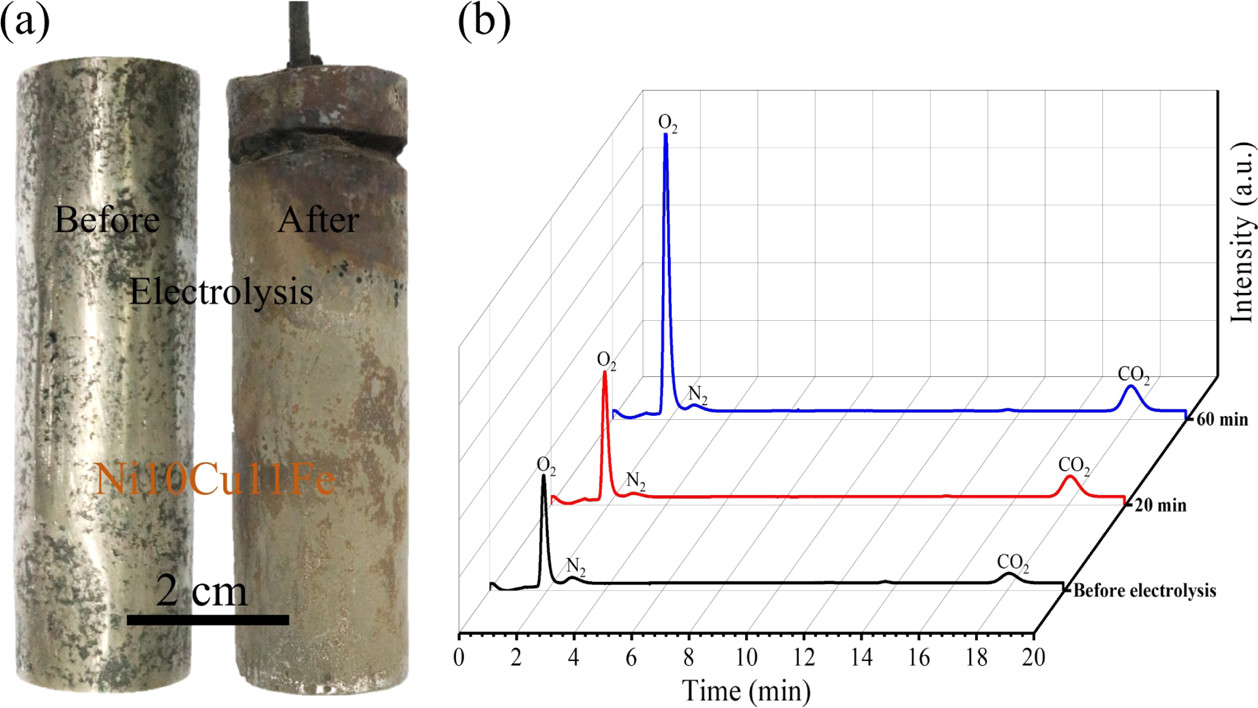
The caption:
Next the solubility of the various oxides, calcium, strontium, and barium were determined by the simple expedient of putting pellets of these oxides in the melts and observing the amount dissolved:
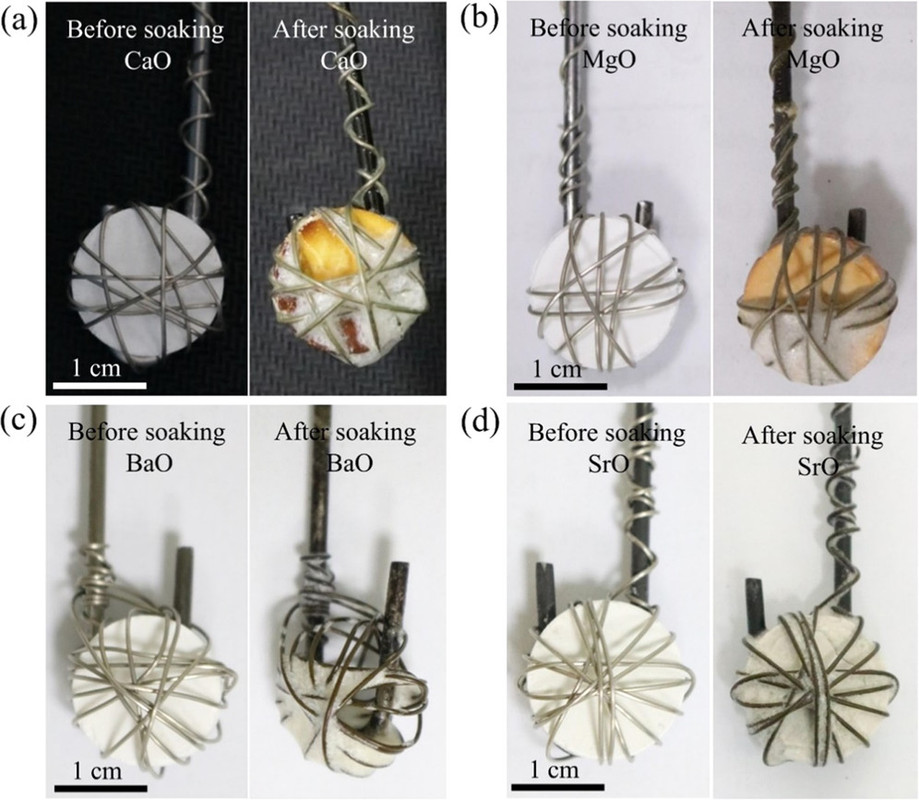
The caption:
Quantitative tables of the solubility of these oxides are not given in the paper, but the general conclusion was that barium oxide was the most soluble and the solubility of its oxide and carbonate were evaluated in more detail along with the rate of dissolution, as shown in the following graphic:
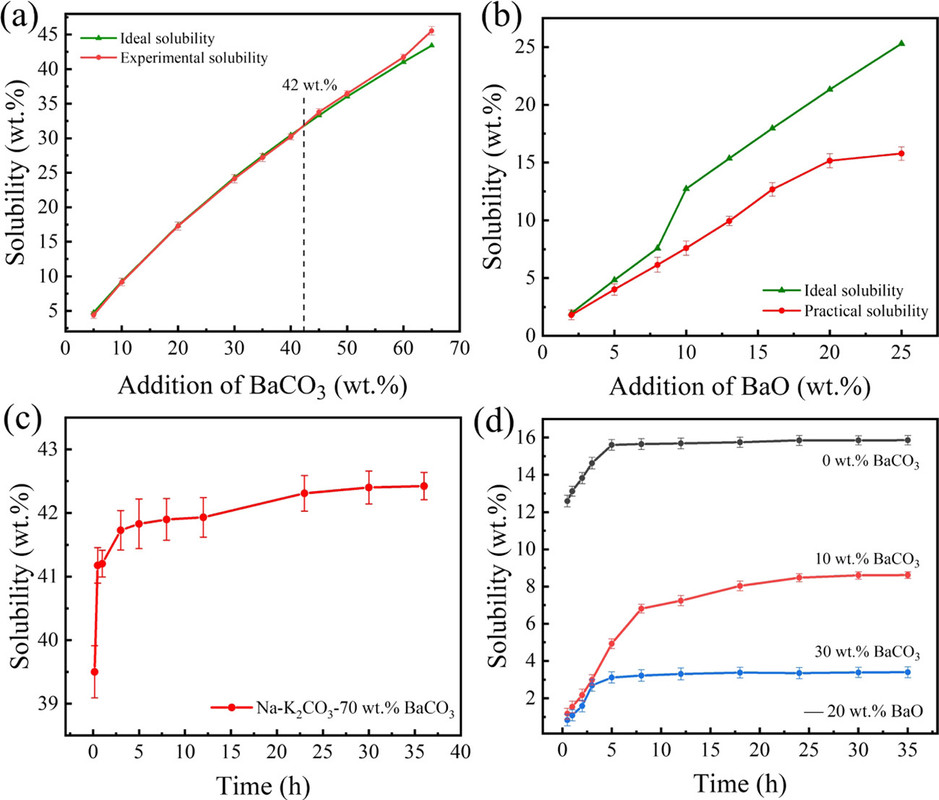
The caption:
It would be nice, from my perspective, if a more complex carbonate system allowing for the use of strontium in this system were considered in subsequent optimization, since strontium is available from used nuclear fuel with a heat generating isotope, Sr-90, which might defray the environmental and economic cost of maintaining a melt, although there are many heat network setting which might address this problem at an acceptable environmental and economic cost.
In any case, the barium oxide can absorb gaseous oxygen, even at these high temperatures - the decomposition temperate of barium carbonate is around 1300°C.
If the goal is, however, to produce the oxides themselves of strontium and/or calcium - the latter oxide is a key constituent of concrete, concrete production being a major contributor to climate change, the insolubility of the oxides is actually a desirable outcome.
The authors write:
This figure obviates that point:
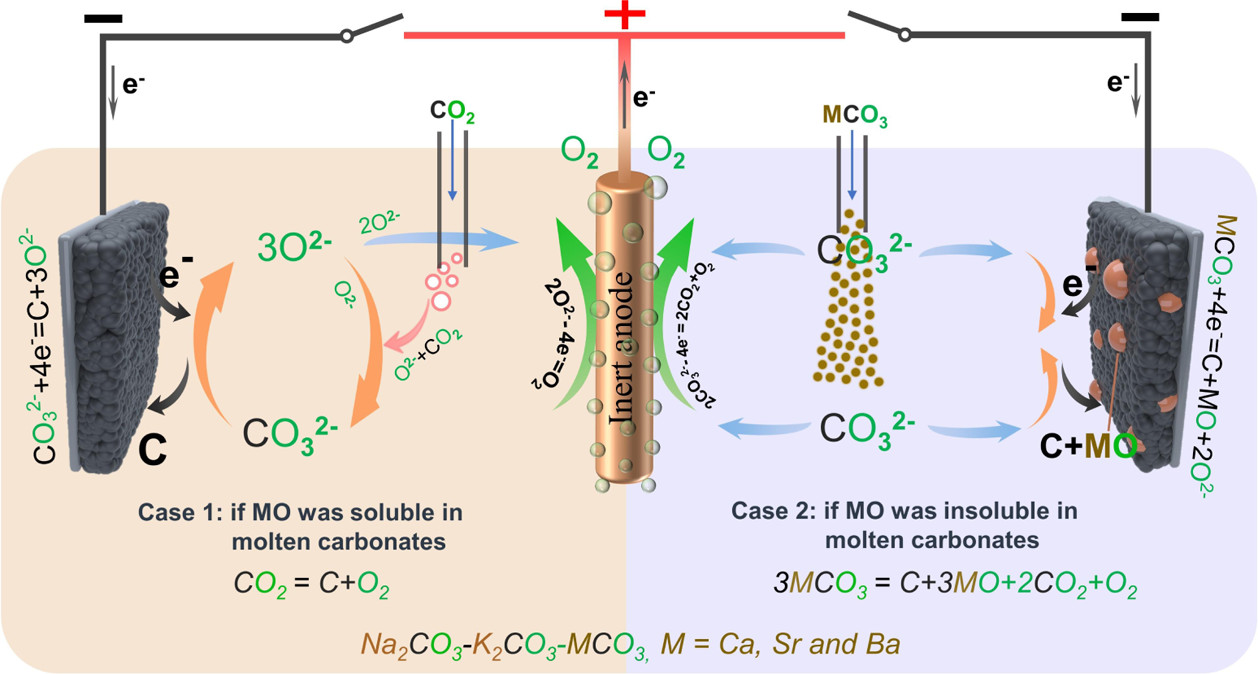
The caption:
They then write about capturing carbon dioxide from flue gas; if this gas is formed to generate electricity, they are then talking about a perpetual motion machine, but that doesn't totally negate the value of the paper itself.
I began this commentary by noting that electricity is a thermally degraded form of energy, but did not state the caveat that electricity captured by use of waste heat that would otherwise be rejected to the atmosphere is merely an improvement in overall energy efficiency. In the case where electricity is generated as a side product, using waste heat from a very high temperature process, the second law of thermodynamics is still operative - there is no physical way to make it inoperative - and increased exergy is obtained.
The conceit surrounding the so called "renewable energy" fantasy is that the intermittent nature of electricity can be addressed by net metering. On the left, we like to mock the statements of the assholes running ERCOT and the racist governor of Texas that the recent events in that State that led Senator Cancun Cruz to run away, the collapse of its power system was an outgrown of the so called "Green New Deal." To be perfectly clear and unambiguous, even as a lifelong Democrat, I don't think that the "Green New Deal" is going to be green or much of a deal. I have zero respect for the tired and old, and frankly experimentally failed energy ideas of Ed Markey, whether I otherwise agree with his other politics, as I often do. So called "renewable energy" is failing us now, and worse, is failing the future. It didn't work to address climate change. It isn't working to address climate change. It won't work to address climate change. Period. A key concept in the ideology of Green New Dealism is so called "net metering" which is the idea that the use of electricity follows its availability, its availability determining its price. If we are honest with ourselves, not that it is easy for anyone to be honest with themselves when honest confronts faith, including quasi religious faith, even absurd faiths, the extreme utility bills seen by some citizens of Texas are "net metering" run wild.
A heat network run by a high temperature nuclear plant, one producing temperatures high enough thermochemically split carbon dioxide - the cerium cycle running at maximum temperatures of 1400°C - will achieve the highest efficiency - the yield of exergy - as a constituent of a heat network, possibly producing a number of Brayton, Rankine and even Sterling cycles in sequence. If this is the case, electricity may be inevitably a side product. If this electricity is used to run combustion in reverse, which is what this paper is all about, the electricity can be switched to the grid whenever electricity prices rise high enough to justify the switching. In effect, a chemical plant is run as spinning reserve. This is not a new idea. Kaiser aluminum - aluminum production is an chemoelectric process - did this historically with hydro power in the Pacific Northwest.
Power demand on the grid fluctuates regularly, with the highest demand in the late afternoon and early evening, precisely when the sun goes down ironically enough, since so many people actually believe that solar electricity will save the world. It won't. It hasn't. Exploiting these fluctuations can be utilized to produce molecular carbon from CO2 gas or provide electricity to the grid, depending entirely on the value to the operator of the plant.
From the paper's conclusion:
It's a very nice paper. I like it a lot.
I trust you are enjoying your weekend.
Use of a Modified SIRD Model to Analyze COVID-19 Data
All of the scientific publishers have made all Covid-19 related papers open sourced, so there is no need to discuss this paper in any detail as interested parties can read it themselves, but here it is: Use of a Modified SIRD Model to Analyze COVID-19 Data (Devosmita Sen and Debasis Sen Industrial & Engineering Chemistry Research 2021 60 (11), 4251-4260.)
Here, anyway, is an excerpt including the definition of "SIRD:"
ARTICLE SECTIONSJump To
Since the beginning of 2020, the whole world has been experiencing a major and unprecedented global crisis, owing to the outbreak of the COVID-19 pandemic.(1?3) The infection is resulting in severe, and sometimes even fatal, respiratory diseases such as acute respiratory distress syndrome.(4) Such an infectious disease, with a humongous social and economic impact was never seen before, at least in the recent past.(5) COVID-19 arose due to a strain of a novel coronavirus that has rapidly spread throughout the globe,(1,6) originating from and infecting a large number of people in Wuhan, China.(7?9) The spread of this disease has a complex time dependence, which is governed not by the number of infected people alone, but is strongly correlated with aspects such as total population of the country, various norms and measures taken by the nation at a particular time, and many more.(10?14) Because of a lack of previous experience in controlling a similar pandemic with such a high impact in the recent past, it is difficult to anticipate the size of the population that may get affected by this pandemic and the typical time required for its control.
The abovementioned crisis immediately calls for a quantitative understanding of the time evolution of this complex and non-linear process through computer modeling. Statistical and mathematical analysis of reported data can provide valuable insights into the trend of the spread and thus can assist in planning various social measures to contain the spread of the virus as quickly as possible. Further, analysis of reported epidemic data plays a vital role in analyzing the underlying phenomena involved in spreading of the disease and to make predictions about future trends. This enables various organizations to efficiently plan their steps toward containing this spread.
In literature, a few models have been proposed to explain such data. These can primarily be classified into two categories, collective models(12,13,15?18) and networked models.(19?23) Some examples of the former class of models are growth and logistic models,(12) the susceptible–infected–recovered–dead (SIRD) model, and their modifications,(9,16) collectively termed as compartmental models. At this juncture, it is worthy to mention that such a phenomenon has a close resemblance to kinetics of chemical reactions in general,(24) where transition from one state to another is associated with a specific rate. In epidemic modeling, the corresponding rates may be expressed in terms of the instantaneous number of infected, recovered people, and so forth. Owing to the complex interdependence of several processes governing the spread of infections and recovery, a proper mathematical model should be able to simultaneously predict the temporal behavior of infected, recovered, and dead people. As the protection procedures demand quarantine, confinement, social distancing lockdown measures, and so forth,(26) a simple SIRD model only gives a preliminary understanding of the process and is not sufficient to describe such complex processes in general. Here, we model the spread of the present pandemic using a generalized SIRD model, taking into account the fraction of population which is exposed, under quarantine, confined, active-infected, recovered, and expired at time instant t. It is noteworthy that proper modeling calls for a simultaneous corroboration of the time evolution of all the three independent reported data sets, namely, active number of infections and cumulative number of recovered and expired people due to the infection.
In this manuscript, we report the analysis of COVID-19 time series data for five countries—China, Italy, France, the United States, and India (and two of its highly affected states)—using the modified SIRD model. We have shown that this model is capable of explaining the current data significantly well for all these five countries. It should be noted that the present approach is unique because it is capable of explaining simultaneously all the three reported data sets: active, recovered, and dead population. The data have been obtained from https://data.humdata.org/dataset/novel-coronavirus-2019-ncov-cases which is compiled by the Johns Hopkins University Center for Systems Science and Engineering (JHU CCSE) from various sources including the World Health Organization (WHO) and from reported data on Indian state websites https://arogya.maharashtra.gov.in/1175/Novel--Corona-Virus, https://gujcovid19.gujarat.gov.in/.
A picture:

The caption:
Enjoy if interested.
An obscure fact I never knew: All of the lanthanides except promethium exhibit a divalent state.
I was wandering around the scientific literature today and came across this very cool fact:
I added the bold.
Trimethylsilyl versus Bis(trimethylsilyl) Substitution in Tris(cyclopentadienyl) Complexes of La, Ce, and Pr: Comparison of Structure, Magnetic Properties, and Reactivity (Chad T. Palumbo, Lucy E. Darago, Cory J. Windorff, Joseph W. Ziller, and William J. Evans, Organometallics 2018 37 (6), 900-905)

Most surprising in this equation is the fact that this holds true for three actinides, thorium, uranium and plutonium. Weird.
I'm glad I found that out before I died.
I was going to plant my summer vegetables and herbs seeds this weekend, but...
...we're having a midweek freeze here in central New Jersey.
That sucks. It's beautiful weather today.
Beto O'Rourke: Leadership on the Front Lines
This is a virtual online event. If you sign up and are unable to attend at the time it runs, they usually send you a recording.
Sign up here for the March 29 event: Tuck Life Long Learning.
One scientist showing appreciation of another in the comments section of a journal.
We often don't think of the social aspects of a scientific life, but in fact, all human strengths and flaws, particularly those involved simply with personality are there. It is a pleasure to work with most scientists, but it is also true that some are oppressive cranks with a nasty edge, including, to be sure, arrogance, contempt and, in many cases, a sense of malignant superiority and dismissiveness.
Of course, most people can display these flaws from time to time; I'm hardly immune myself.
At the end of most issues of scientific journals there'll often be short section in which comments on previous papers are offered. This is particularly true of ACS Journals, which tend to dominate my reading.
Often these comments are critical of the published paper under discussion, sometimes highly critical, even to the point of barely disguised hostility, even contempt.
I was very pleased to see one today that praised a paper and then simply asked for more information.
It's here:
Comment: The Novelty of a Two-Step Aromatization Process (Pulkit Bajwa, Industrial & Engineering Chemistry Research 2021 60 (10), 4189-4190)
An excerpt:
...A design of experiments strategy was briefly mentioned in the beginning of section 3.2 of this article. Statistical analysis was not provided in the Supporting Information. Variations in reaction conditions may significantly change the outcome of the reaction. As explained in the other study(2) interactions between factors generally provide additional important information. Was this true for this two-step study? Statistical details such as main effects, interactions, or contour plots could reveal more information on this topic, particularly the second step. Therefore, I would like to ask for more information on statistical analysis and answers to questions that are asked above. Researchers would be interested in this information, which will certainly add value to the current study. Perhaps it would encourage engineers and scientists to conduct more detailed studies on aromatization of hydrocarbons from renewable sources and coke characterization, and use statistical data for optimization.
"Design of Experiments," often abbreviated as "DOE" is a statistical approach to evaluating parameters in a process to understand how these parameters interact to delineate a path to a desired outcome.
After these kind remarks, the authors of the paper under discussion replied:
Reply to “The Novelty of a Two-Step Aromatization Process” (Swapnil Fegade, Brian Tande, Alena Kubátová, Wayne Seames, and Evguenii Kozliak Industrial & Engineering Chemistry Research 2021 60 (10), 4191-4191)
Further information about the statistical work performed during the study is available in Fegade(1) appendix C which contains all of the raw statistical data generated as well as additional statistical results from the Minitab program. These details were not included in the paper due to publication space limitations. Additional details can also be found in a patent by Seames and Tande.(2)
I can certainly appreciate when scientists "greatly appreciate" each other and be helpful to one another.
Science is a very human activity, and it's a joy when science can include graciousness. This is not always possible, but when it is, and is exercised, I certainly applaud it.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,512