General Discussion
Related: Editorials & Other Articles, Issue Forums, Alliance Forums, Region ForumsWe Need to Talk About the AstraZeneca Vaccine
For the moment, reports of a very rare, dangerous blood disorder among recipients cannot be ignored.
https://www.theatlantic.com/health/archive/2021/03/astrazeneca-vaccine-blood-clot-issue-wont-go-away/618451/

The AstraZeneca COVID-19 vaccine is indispensable right now. As one of the first vaccines out of the gate, it’s been at the centre of the World Health Organization’s plan to roll out some 2 billion doses to 92 nations by the end of the year. It’s also one of just a handful of vaccines that are already being produced and distributed on such a massive scale that they might change the near-term course of the pandemic. That’s why the past few weeks have felt so catastrophic. The run of bad news might have seemed, at first, to be short-lived. Earlier this month, regulators in more than 20 European countries suspended distribution of the AstraZeneca vaccine. The English-language media cited scattered reports of “blood clots” in recipients as the reason. A few days later, though, the European Medicines Agency’s expert committee weighed in to recommend the vaccine’s continued use. With COVID-19 case rates surging across Europe and more than 3,000 deaths a day, the group concluded that its benefits far outweighed any known or potential risks.
Just that short pause sparked despair and condemnation. Commentators and public-health experts called it “stupid, harmful, “quite dangerous,” and a “magnificent example of European failure.” The problem, they said, was that the actual evidence of harm had been very weak—and maybe even non-existent. Writing in The New York Times on March 22, Heidi Larson, the director of the Vaccine Confidence Project at the London School of Hygiene & Tropical Medicine, noted that just 25 Europeans had developed blood clots, out of 20 million who received the AstraZeneca vaccine. That rate, she said, was lower than what you’d normally see among unvaccinated people. According to the statistician David Spiegelhalter, the furore over blood clots showed our “basic and often creative urge to find patterns even where none exist.”
None of these critics said that potential risks should be ignored. They argued instead that, given the available data, the known harms from COVID-19 were clearly many orders of magnitude more significant. The cost of losing time from a temporary pause in vaccination was therefore disproportionate and unbearable; worse, it was likely to exacerbate concerns among vaccine-wary Europeans. Indeed, close to 60 percent of French adults now say they have little or no confidence in the AstraZeneca vaccine; similar poll numbers are turning up in Germany, Italy, and Spain. As Larson suggested in her op-ed last week, the AstraZeneca vaccine may now be back in distribution in many places, but the drama has “heightened anxieties and increased hesitancy.” That effect could spread well beyond Europe, and beyond this particular vaccine.
But the challenges here are far deeper than this blizzard of commentary allows. The risk of a dangerous vaccine reaction could be very real, if also very rare—and major European vaccine authorities have not, in fact, been overcautious, political, or innumerate in responding to this possibility. Rather, they’ve been faced with something of a nightmare scenario for vaccine communication. We’re in the midst of a global public-health crisis, and regulators must address the possibility (still unproved) that perhaps one in every 1 million vaccinated people could have a potentially fatal drug reaction—as more than 1 million vaccine doses are being injected each day in Europe alone. It seems as though anything the regulators say about this problem could serve to reduce trust in vaccination, and thus increase the toll of the pandemic. And yet if there does turn out to be a vaccine reaction, even a vanishingly infrequent one, keeping mum won’t make the problem go away. Indeed, it could serve to worsen the effects of the fearmongering about vaccines that will surely grow from here.
snip
AstraZeneca Vaccine Fails To Protect Against The South African Variant, Says Study
https://www.google.com/amp/s/www.forbes.com/sites/williamhaseltine/2021/03/17/astrazeneca-vaccine-fails-to-protect-against-the-south-african-variant/amp/
Two doses of the AstraZeneca Covid-19 vaccine were found to have only a 10.4% efficacy against mild-to-moderate infections caused by the B.1.351 South Africa variant, according to a phase 1b-2 clinical trial published on Tuesday in the New England Journal of Medicine. This is a cause for grave concern as the South African variants share similar mutations to the other variants leaving those vaccinated with the AstraZeneca vaccine potentially exposed to multiple variants. This new finding should force a rapid acceleration of second-generation vaccines and encourage further research into the possibility of a pancoronavirus vaccine.
The trial evaluated the safety and the efficacy of the AstraZeneca vaccine in HIV-negative adults aged between 18 to 64 years old with a median age of 30 years old. The trial was conducted between June 24 and November 9, 2020 in South Africa using a multisite, double-blind, randomized, placebo-controlled approach. Out of the trial’s 750 vaccine recipients, 19 (2.5%) developed mild to moderate COVID-19 more than 14 days after the second dose, compared with 23 of 717 placebo recipients (3.2%). Of the 42 total cases of Covid-19, 39 (93%) were caused by the B.1.351 South Africa variant. These results demonstrated that the AstraZeneca vaccine was only 10.4% effective against the B.1.351 South Africa variant. It is important to note that there were still no cases of hospitalization for severe Covid-19 or deaths observed in the study. Yet the authors did caution that the relatively young median age of participants (30 years) likely influenced the lack of severe Covid-19 cases.
The South African B.1.351 shares similar mutations with several other variants. Mutations to positions 417 (K417N), 484 (E484K), and 501 (N501Y) are all located in the receptor-binding domain. This structure is the part of the spike protein that attaches to the ACE2 receptor of the human cell. The K417N and E484K mutations have been seen in the Brazilian and Japanese variants, and N501Y has additionally been seen in the UK variant. External to the spike protein, there are a set of three deletions in non-structural protein six which also appear in the Brazilian, Japanese, UK, Nigerian, and New York variants. NSP6 is a structural transmembrane protein and these deletions additionally may assist in neutralization escape. NSP2 also carries a common mutation: T85I.
This mutation appears in the California variant, the New York variant, and a number of other US variants. While NSP2 has no known function, the pervasiveness of the mutation is notable at the very least. In NSP12, mutation P323L is pervasive in nearly every variant. This protein is the polymerase, which controls viral replication. While it may not aid immune-escape, this mutation certainly aids increased transmissibility of the South African variant and others. Suffice to say, despite these variants carrying unique sets of mutations, individual changes are shared across lineages that may aid to the neutralization escape the South African variant demonstrates. As these variants threaten to become the dominant source of coronavirus cases globally, we urgently need second generation vaccines that provide greater protection against the variants if we are going to prevent another wave of infections and return to a level of normalcy. The UK B.1.351 variant and NYC variant B.1.5.26 are now responsible for over 51% of New York Covid-19 cases.
snip
LisaL
(47,365 posts)The ultimate goal of all of them (whichever technique they use) is to deliver covid spike proteins to cells. What would cause AstraZeneca to have the clotting reaction?
OnDoutside
(20,862 posts)in Astrazeneca the company. It's been one thing after another with them, and that's really what has caused the collapse in confidence amongst Europeans. You never really hear bad news stories about the Pfizer vaccine do you ? I'm pretty sure that I am due to get the Astrazeneca vaccine, whenever it turns up. I'll take it but I wish it was one of the other vaccines.
is the first and only widely distributed vaccine in history which uses an adenovirus to deliver its payload. The AZ uses a chimp virus, and J&J uses a uncommon human virus.
It could have nothing to do with this, but this isn't an old vaccine in an old delivery system that is well known.
Additionally, this is a very rare (although not unknown in other viral infections) side effect.
Tree Lady
(13,107 posts)Any pain medication aspirin etc when she had reaction from Moderna because of blood clots.
I had no idea what she was talking about.
I took Tylenol no problem with Moderna shot after.
LisaL
(47,365 posts)NT
Leith
(7,864 posts)Aspirin keeps blood from clotting - it does not cause or facilitate clotting.
Tree Lady
(13,107 posts)What she was talking about. I am going to see her next month and will ask.
Leith
(7,864 posts)Your friend's reasons may have been a bit garbled in communication, but it looks like she heard the same thing that several DUers have heard.
muriel_volestrangler
(105,836 posts)though that's not about blood clotting.
"If you have mild symptoms just after getting the vaccination, try to tough it out. If you can’t tolerate the temporary discomfort, I would use acetaminophen (Tylenol) over an NSAID,” Craig B. Wilen, MD, PhD, an immunobiologist in the Department of Laboratory Medicine at Yale University School of Medicine and a lead researcher from the study, tells Verywell. “Our study was early so there are no firm recommendations, but based on other studies of NSAID use with vaccinations, the theory is that NSAIDs decrease the antibody response.”
https://www.verywellhealth.com/pain-reliever-covid-19-vaccine-5111319
I don't know if there's been more research since then.
marble falls
(71,399 posts)Celerity
(54,005 posts)Blue_true
(31,261 posts)Any actionable data yet? Or is it too early for the particular vaccine study phase that you are now part of?
Celerity
(54,005 posts)to report so far.
We had very mild sore arms that went away the next day. Nothing else.
Blue_true
(31,261 posts)marble falls
(71,399 posts)malaise
(294,198 posts)No side effects here. Didn't have any pain in my arm either but again I used the icepack twice a day for the first two days.
I take an 81 aspirin once a day but the doctors told all of us to take Panadol every six hours for 48 hours after the vaccine. After that I returned to my daily baby aspirin.
Fiendish Thingy
(22,460 posts)It gives emphasis to the clotting issue, something that the article seeks to allay concern about.
The article is more concerned about the primarily emotional, rather than data-driven, reaction by European health agencies, and the subsequent distortions in the media. Astra Zeneca deserves its share of criticism, but the clotting issue is so rare (25 cases out of 17 million injections), that it wouldn’t be expected to show up in a trial of just 35,000 subjects.
Astra Zeneca deserves criticism for the unorthodox way they merged data from trial under differing conditions; a more conventional trial has just been completed, and hopefully that data will permit FDA authorization in the US.
In the meantime, Biden has sent 1.5 million doses of AZ to Canada (thanks, Joe!), which will allow the acceleration of our vaccination program.
I have just found out that, by the end of next week, BC will start vaccinating 55-65 year olds (they’re currently limiting vaccines to age 72+). This is 2-3 weeks ahead of schedule, which means I should get to see my adult kids that much sooner!
LisaL
(47,365 posts)We have three other vaccines, and should have more than enough doses for everybody. Not sure what adding AstraZeneca to the other three going to accomplish?
As for blood clot side effect being rare, yes, it's rare, but it's serious and have caused people to die.
Blue_true
(31,261 posts)The Novavax vaccine can be a game changer, because it can be stored in refrigerators that pharmacies are already using to store Flu vaccines, and doesn’t need to go through a thaw cycle (from which the vaccine can’t be used if it doesn’t get into arms).
Celerity
(54,005 posts)https://www.macleans.ca/news/15-questions-about-the-astrazeneca-vaccine-in-wake-of-new-recommendations/

In the past few months, as AstraZeneca got its COVID-19 vaccine approved in Britain, Canada and Europe, it has contended with a growing list of concerns about its research and marketing techniques as well as deepening worries about whether the vaccine is causing blood clots, including obstructions that prevent blood from draining from the brain (known as cerebral venous sinus thrombosis), which killed at least nine people in Germany shortly after they received the AstraZeneca vaccine. On Monday, Canada’s National Advisory Committee on Immunizations (NACI) altered its guidance regarding the AstraZeneca COVID-19 vaccine and recommended a pause in giving it to adults under 55 years of age until more investigations could occur into a possible relationship between the vaccine and blood clots.
Link to tweet
Didn’t the European Medicines Agency just review that blood clot concern and say the vaccine was safe?
Earlier in March, as reports of blood clots were being flagged in the surveillance systems that monitor any possible post-vaccination side effects, more than 20 European countries temporarily stopped using the AstraZeneca vaccine. At that point, there were 37 reports of blood clots among the more than 17 million people who received doses in Europe and Britain. On March 18, the European Medicines Agency (EMA) stated that the AstraZeneca COVID-19 vaccine “is not associated with an increase in the overall risk of blood clots.” Still, while the EMA committee conducting the preliminary review “was of the opinion that the vaccine’s proven efficacy in preventing hospitalization and death from COVID-19 outweighs the extremely small likelihood” of developing two rare blood clotting disorders, it also cautioned that the vaccine may be associated with very rare cases of blood clots associated with low levels of blood platelets.”
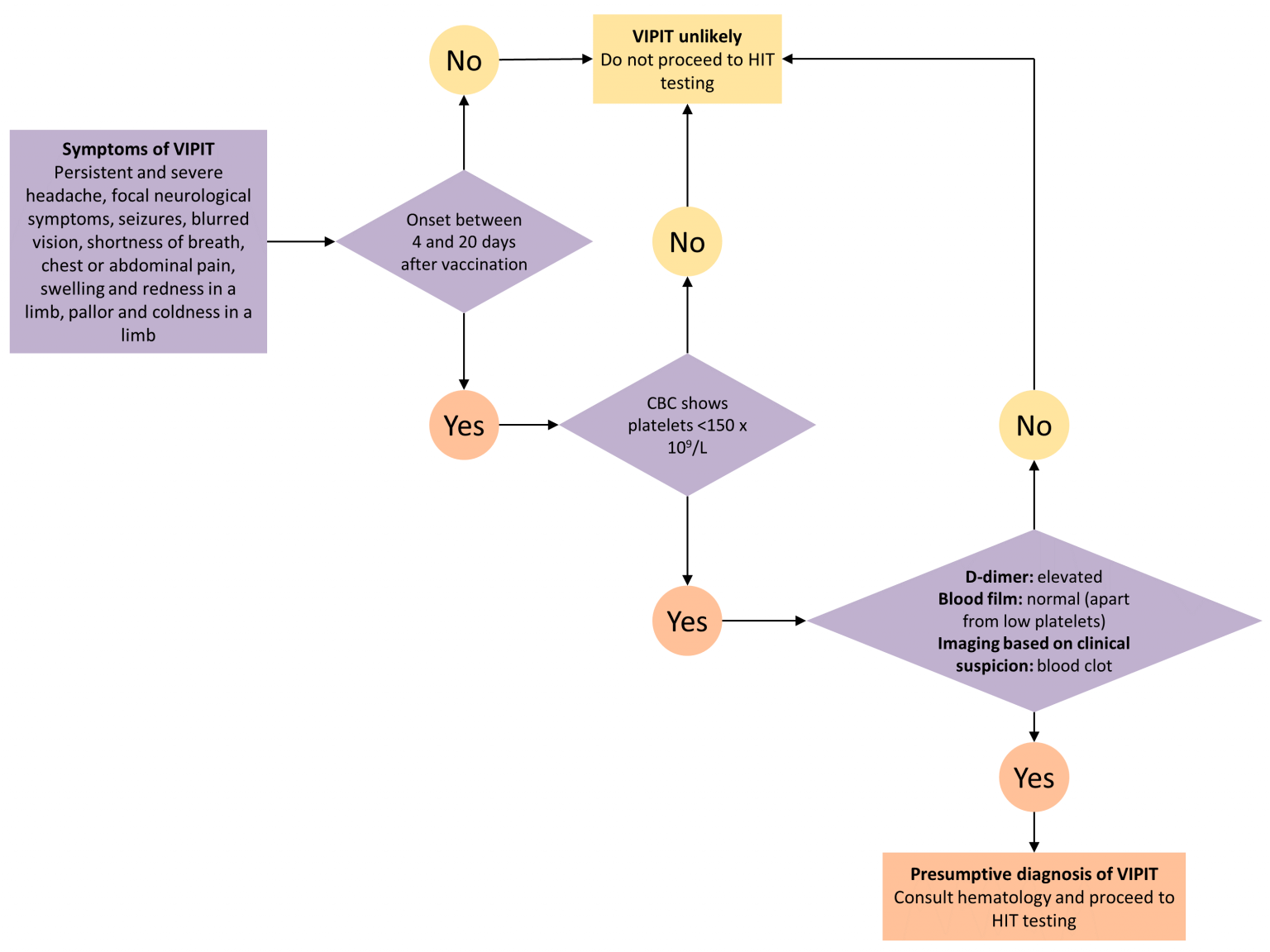
What changed since that EMA decision and what is VIPIT?
Research into the clotting issue didn’t stop, especially into that unusual combination of people having widespread blood clots and low platelet counts, which resembles “a rare side effect of the blood thinner heparin called heparin-induced thrombocytopenia,” Science magazine reported. Clotting specialist Andreas Greinacher named that combination of symptoms “vaccine-induced prothrombotic immune thrombocytopenia,” or VIPIT. And, as Science explains, though other researchers weren’t sure of Greinacher’s explanation, they were convinced that the vaccine was causing rare symptoms. A haematologist in the United States told The Atlantic that while he wasn’t yet convinced by the “vaccine-induced” part of the VIPIT name, he did think “these cases raise concern that this vaccine is potentially life-threatening in a small subset of patients.”
How common is VIPIT among those who have received the AstraZeneca vaccine and why are those clots concerning enough for NACI to change its recommendation?
The Ontario COVID-19 Science Advisory Table offers a lay summary: “These blood clots have two important features: they occur four to 20 days after vaccination, and they are associated with low platelets (tiny blood cells that help form blood clots to stop bleeding).” Research is still very early. Scientists had found at least 13 people with both blood clots as well as low platelet counts before the EMA decision, according to [iScience magazine]. And that later research, led by Greinacher, looked at only nine people who exhibited blood clotting events after vaccination (seven had cerebral venous sinus thrombosis (CVST); another had CVST plus another vein thrombosis; the ninth had a pulmonary embolism; four of them died), and examined the blood samples that were available for four of them.
Most of the cases of VIPIT in Europe involve women under 55 years of age, though that may be the result of vaccines being given to more women than men. Right now, according to NACI, “the case fatality of VIPIT is approximately 40 per cent,” though that the rate may fall with more awareness of the potential side effect and earlier treatment. While scientists need to conduct more research into establishing whether there is a definite link between VIPIT and the AstraZeneca vaccine, NACI felt there was enough new data that it wanted to revise its recommendation, which it did on Monday. To date, no cases of VIPIT have been reported in Canada. As of now, “VIPIT seems to be rare, occurring in anywhere from 1 in every 125,000 to 1 in 1 million people,” the Science Table stated in its March 26 guidance, though those statistics are being constantly updated. On Monday, the Paul-Ehrlich-Institut released more surveillance data for Germany: of around 2.7 million people given the AstraZeneca vaccine, there have been 31 cases of CVST (29 were women), with nine deaths. How many of those cases of cerebral blood clots also have low platelet counts isn’t yet known.
Link to tweet
snip
Fiendish Thingy
(22,460 posts)LisaL
(47,365 posts)VIPIT stands for vaccine induced prothrombotic immune thrombocytopenia. Since it's vaccine induced by definition, your claim isn't making sense.
beat me to it, lol
Celerity
(54,005 posts)as VIPIT is vaccine-induced
Fiendish Thingy
(22,460 posts)Compared to clotting conditions such as DIC in the general population- is that clearer?
Celerity
(54,005 posts)so your claim
is utterly illogical
https://covid19-sciencetable.ca/sciencebrief/vaccine-induced-prothrombotic-immune-thrombocytopenia-vipit-following-astrazeneca-covid-19-vaccination-interim-guidance-for-healthcare-professionals-in-emergency-department-and-inpatient-settings/

Fiendish Thingy
(22,460 posts)By the time of Vogel and Kupferschmidt’s first article, though, the prothrombotic immune thrombocytopenia problem had already been recast in the English-language media as simply one of “blood clots.” Seen in that context, the decisions to suspend the AstraZeneca rollout were puzzling—and perhaps, as some maintained, driven more by emotions than by data. The European Medicines Agency had helped create this impression, starting with a press release on March 10. In its statement, the agency compared the number of vaccinated people who had blood clots with the baseline rate across the population. Commentators quickly zeroed in on that comparison, or a similar one from an AstraZeneca press release, which stated that the number of events “is much lower than would be expected to occur naturally in a general population of this size.”
Celerity
(54,005 posts)You falsely accused me of 'forgetting' things (a negatively loaded implicature when used in such manner and fashion as you did) when I didn't write the articles, and I post under copyright TOS constraints on this board.
Your use of grammatical syntax, formulation, and linguistics was so flawed that you litterally constructed a self-nullifying argument.
You persisted even after I presented you with an official government health agency-produced diagnostic tree, plus the link to a more granular explanation in terms of what constitutes VIPIT and what rules it out at present.
You also completely ignored the extremely low (10.4 % so far) efficacy level for the AZ vax against the surging B.1.351 variant, another, and bigger issue with the vax.
My posts and replies all stand. Done here.
Cheers
NotANeocon
(465 posts)It would seem there is a particularly vicious campaign against the Brit vaccine despite the fact it will save millions of lives. Capitalism has only one aim -and it aint the welfare of the people.
ornotna
(11,435 posts)NPR
South Africa is going to sell all their doses because it's not as effective on the SA variant.
Celerity
(54,005 posts)so I do not see a huge market, especially when combined with its other problematic issue atm.
Ace Rothstein
(3,369 posts)We also don't need it. We'll have enough of the other vaccines by late spring.
speak easy
(12,595 posts)They knew it was the least effective, with the most complications, but they wanted 'value for money'.
The UK, on the other hand 'opened their wallets
The EU’s vaccine debacle
https://timesofmalta.com/articles/view/the-eus-vaccine-debacle.851597
LisaL
(47,365 posts)It works well against the original variant and UK variant. UK is using it and their rates of covid dropped way down.
