Environment & Energy
Related: About this forumA Detailed Thermodynamic Accounting of a Route to Obtaining World Motor Fuels from Solar and Wind.
(Edited 2/9/22 to restore graphic links. Since the original version of this post was completed, the two papers listed immediately below have been corrected.)
The main papers I'll discuss, among others, in this post are these:
Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide—Part I: Modeling and Analysis for OME1 (Mitsos et al, Ind. Eng. Chem. Res., 2019, 58 (12), pp 4881–4889)
Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide—Part II: Modeling and Analysis for OME3–5 (Mitsos et al, Ind. Eng. Chem. Res., 2019, 58 (14), pp 5567–5578)
Here is a swell graphic from the introduction of one of these papers:
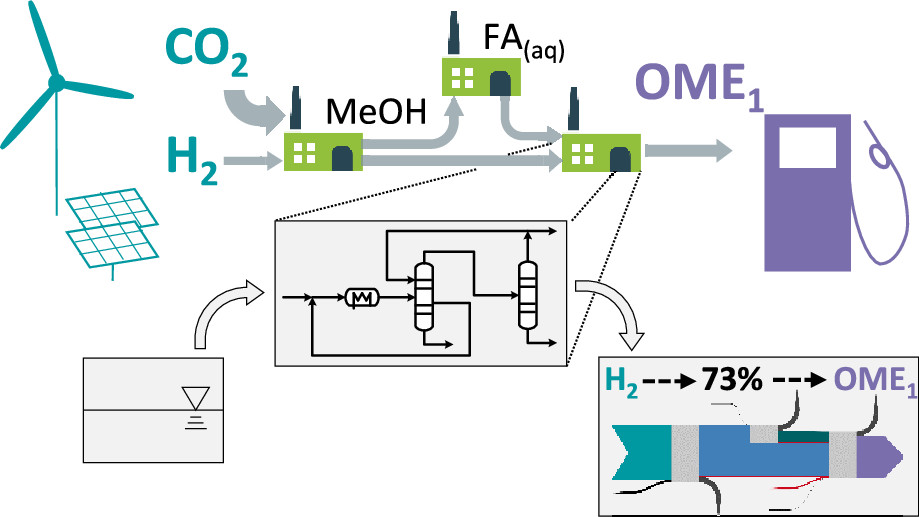
It's, um, a cartoon.
The storage of energy, in general, wastes energy, and the amount of energy wasted by batteries, for example, is quantifiable in terms of particular technologies. This irrefutable fact is connected with the second law of thermodynamics, a law which cannot be repealed either by a tin-horn dictator, a legislature, a prime minister, president, by anyone. It is a law of physics and not social or political "science."
This was the topic - how much different types of batteries waste - I covered in this space in my most recent post: The External Cost, Including Climate Cost, of Stationary Batteries For Grid and Off Grid Power.
Probably that post was too "sciency" - as this one will be - and hardly as sexy as a post based on a university press release announcing a "breakthrough" involving some pop fantasy about electric cars, windmills and solar cells, said fantasies having done nothing at all, after half a century of delusional cheering, to arrest the growth in the use of dangerous fossil fuels or the growth in environmental destruction caused by them, most notably, climate change.
In general, I am a wild-eyed critic - a sort of screaming and chanting wild-eyed prophet in a desert - of the car CULTure, but to demonstrate an important caveat about the waste connected with storing energy, I will use the popular "car think" that is familiar to most people, and consider the difference between a hybrid car fueled by dangerous fossil fuels and an fully electric car.
Both types of cars contain large capacity batteries, but the difference between them is connected to the type of electrical energy they store:
A hybrid car of course requires gasoline to run, and with the exception of a few plug in versions like the discontinued Chevy Volt, the electricity stored in the battery is generated by the use, as primary energy, gasoline. Of course, almost all of the cars on the planet still use gasoline, so this makes them different than electric cars. But the energy used to charge the battery in a hybrid car is already waste energy, specifically the energy lost to braking - in a pure gasoline car this energy is wasted as heat generated in the brakes - the energy wasted when the car is stuck in traffic, or idling at a traffic light, and the energy wasted by car's generator which is necessarily in excess to accommodate all permutations of cabin use of electricity for heating & air conditioning, entertainment systems, navigation systems and communication systems such as cell phones and internal automotive "hot spots." (One must love our bourgeois culture, it's so, um, um, um, holy?.) Therefore automotive generators are required to produce more electricity than the average demand, and are in fact designed to meet a putative maximum demand. Any electricity generated by an automotive generator beyond the demand at any particular point is already waste energy, and thus if it is used to charge a battery that is not at capacity it is converted from waste to recovered energy.
The energy conversions involved in a hybrid car are these: Chemical energy is converted to mechanical energy and electrical energy, and mechanical energy and electrical energy are converted back to chemical energy (the battery.) Some of the mechanical energy is recovered in braking, some in idling.
Now let's consider an electric car:
Most people sitting in praise of electric cars like to pretend that all of the energy coming out of their wall socket comes from solar and wind energy. Some people even pay extra money to assure that this is true and get these nice little paper "certifications" of this purported "fact." However, the real fact, is that there are no clerks how sort electrons directed to a particular house. Anyone who believes that this payment has any meaning merely needs to consider if his or her lights, air conditioners, television sets and computers go off on a hot summer night when the wind isn't blowing.
In fact, so called "renewable energy" is a trivial form of energy. Between 2016 and 2017, worldwide energy demand grew by 8.88 exajoules to 584.98 exajoules whereas all the solar, wind, geothermal and tidal crap combined increased it's output by 1.21 exajoules to 10.63 exajoules total. The fastest growing source of energy on this planet was dangerous natural gas, which grew in that one year period by 4.19 exajoules, in the mindless "percent" talk one hears when energy is discussed by partisans of the failed so called "renewable energy" scheme, by 344% faster than so called "renewable energy." Petroleum use rose by 1.97 exajoules in that period, making it the second fastest growing source of energy.
In the United States, an oil and gas drilling hellhole, the proportion of electricity produced by dangerous fossil fuels is growing rapidly, as spectacularly safe nuclear energy is displaced by spectacularly unsafe natural gas, accompanied by absolutely mindless cheering from people who no interest in and no knowledge of science and absolute contempt for the future of humanity.
So when I consider the electric car I will be reality based, even if 40% of the electricity in my state (down from 50%) comes from clean nuclear energy, and remain close to reality in this country as a whole, and practically every other country on this planet, and consider that almost all of the electricity comes from dangerous fossil fuels.
To propel an electric car, chemical energy, in the form of dangerous fossil fuels, is converted to thermal energy, the thermal energy is converted to electrical energy (in the generator), the electrical energy is shipped with some, albeit small, loses to thermal energy, then converted to chemical energy (the battery), than reconverted to electrical energy, and then converted to mechanical energy, (the electric motor). If the electricity is generated by a combined cycle dangerous natural gas plant, the thermal efficiency of the first step is roughly 50%, the electricity delivered at the outlet due to transmission losses is 97%-99% of the generated electricity (depending on distance), depending on the battery type, the charging efficiency is shown by this graphic from my previous post, in which the purple portions of the bar graphs in the LCE headings more or less follow the thermodynamic efficiency of batteries including the cost of generating the electricity:
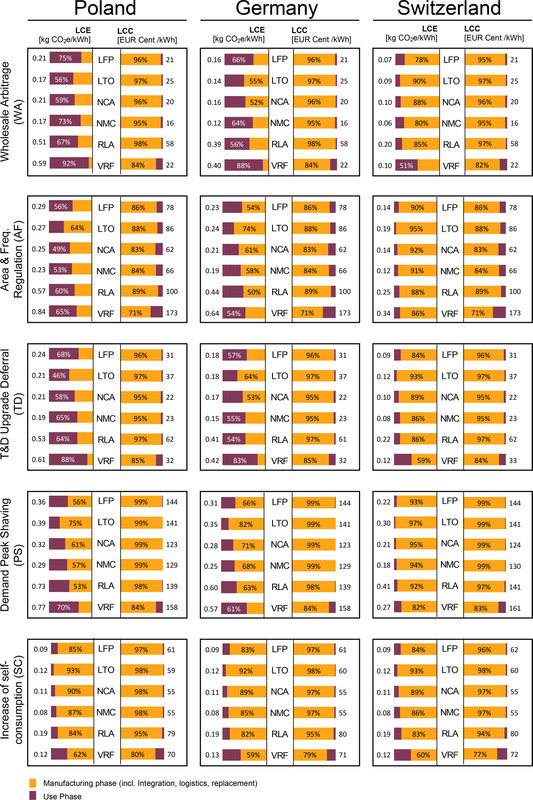
The caption:
(Note that most of the electricity in Switzerland is either generated by nuclear energy (about the same proportion as New Jersey) or hydroelectricity, at least until the glaciers are gone. Switzerland's citizens have voted to phase out nuclear energy. Goodbye glaciers.)
The important point is that with the exception of the mechanical energy converted to chemical energy by braking, none of the energy for charging an electric car is inherently waste energy. All of it comes from primary energy which is converted at huge thermodynamic penalties into mechanical energy.
Since the overwhelming majority of the world's electricity is generated by dangerous fossil fuels, this waste connected with pure electric cars shows up in the planetary atmosphere as carbon dioxide, nitrogen oxides, sulfur oxides, volatile mercury, volatile lead, benzofurans, benzodioxins, PAHs etc, etc, etc.
A hybrid car is not a "green" or "clean" machine, but in many cases, if not New Jersey where I live partially powered by nuclear energy, an electric car is potentially more noxious than a hybrid car, even considering the fact that it's drive train, if not its comfort systems, are not wasting energy at traffic lights or in heavy traffic. This fact is regularly discussed in the primary scientific literature.
But this post is not supposed to be about cars, which, with acknowledged hypocrisy, I drive, but which I nonetheless detest.
It is about chemical fuels that can be produced from waste carbon dioxide using so called “renewable energy.”
This brings me to the paper discussed at the outset.
From my perspective, the most valuable chemical fuel to displace all the world's demand for gasoline, LPG, diesel fuel, dangerous natural gas, and, paradoxically, many refrigerants, is dimethyl ether.
Here is the marketing web page of the International Dimethyl Ether Association listing some of the benefits of this fuel:
International DME Association.
Very often good ideas lack the strength of bad ideas, which is why we are where we are on this planet, but no matter. The technical superiority of DME to all other chemical fuel options is clear enough, but the fact is that DME, like the much ballyhooed hydrogen from which it may be made, is not primary energy. It is stored energy.
It is fashionable, even among scientists, to claim that the goal of almost every effort to make liquid fuels will be a path for storing so called "renewable energy." Probably this is a good way to get grants, given the popular if delusional mentality with which energy is almost always discussed.
However, if one merely looks at reality, rather than continuously reading or pronouncing conditional statements including featuring the word "could," or making rather absurd statements like this one, from the introduction to Part I of the two papers under discussion...
...belief in the viability is ridiculous because we are more than half a century of constant yelping and cheering about so called "renewable energy" and, as of 2017 the combined solar, wind, geothermal and tidal energy schemes were producing 10.63 exajoules out of 584.98 exajoules being generated and consumed by humanity.
The "could" above has been put in bold by me, obviously. I have been hearing the word "could" in connection with so called "renewable energy" so long, I am compelled to wonder why there is never any is.
In another context entirely Abraham Lincoln remarked that...
So called "renewable energy" has not proved it can produce significant electricity, never mind make enough electricity to make stored liquid fuels. The problem is that there is very little renewable energy on this planet, so little that any effort to store it, at a thermodynamic loss no less, would be self defeating, making it even more useless than it already is, and it is useless.
However the good part about all this nonsense is this. Much of what is written with de rigueur genuflections to so called "renewable energy" is actually applicable for energy that is actually sustainable and scalable, nuclear energy. That is certainly the case for these two papers which, after the nonsense statements about so called renewable energy are completed, turns to a very practical consideration, the conversion of the dangerous fossil fuel waste carbon dioxide into a useful product, a fuel for self propelled vehicles such as buses, delivery trucks, ambulances, tractors, etc...
The first two paragraphs of part I of the paper however do proceed with the usual wishful thinking, and include reference the thermodynamic nightmare of water electrolysis, the most environmentally and economically suspect alternative to producing hydrogen from natural gas. About 1% of the world's hydrogen is produced by electrolysis, and then only as a side product of the production of chlorine gas and caustic soda, NaOH.
Nevertheless, despite it's inherent thermodynamic penalties, it is important to note in all discussions in these papers, the authors are basing all of their comments on an assumption that the hydrogen is formed electrolytically, a thermodynamic nightmare, and further that all of the electricity in question comes from the still (and always) useless solar and wind industries.
The third paragraph is the first one worth reading, since it refers to the valuable core of the paper. It reads:
(The "n" here is a subscript in the original paper. The DU editor no longer allows for subscripts.)
The structure of methylal is this:
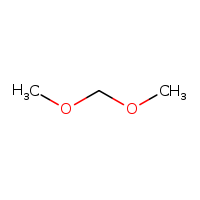
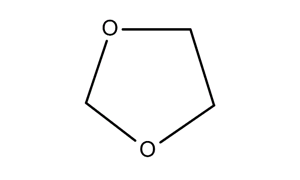
Formally this type of structure is known as an "acetal," which is made by the condensation of an aldehyde and two alcohol functions which may or may not be part of a single molecule. In this case, the aldehyde is formaldehyde, the smallest known aldehyde, and two methanol molecules. Both formaldehyde and methanol can be made by the hydrogenation of carbon dioxide, albeit under different conditions using different catalysts. (There are also electrochemical means of producing formaldehyde from carbonate, but all systems using electricity for reduction in aqueous solution have low thermodynamic efficiency.) Dioxolane is an acetal of formaldehyde and ethylene glycol and is an acetal formed from a diol, a single alcoholic molecule as opposed to two molecules:
Related to acetals are "ketals" in which the carbonyl reacting with the alcoholic functions are ketones rather than aldehyde. Here's a picture of one of my favorite ketals, "soketal" which is made from glycerol, the waste product made from the production of soap and biodiesel, by condensation with acetone:
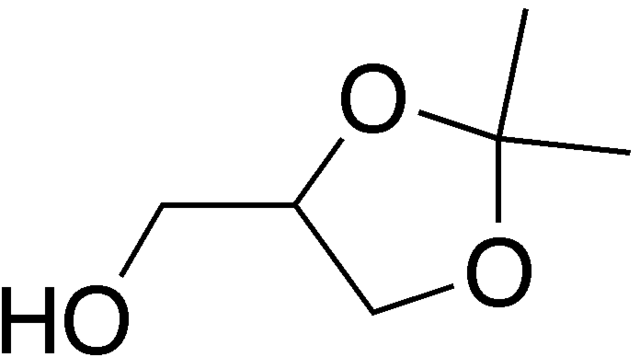
(Soketal, unlike glycerol, has some interesting properties that suggest it's potential use as a fuel.)
In any case, the general structures of the molecules discussed in these two papers is this:
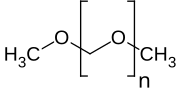
Here the "n" refers to the number of formaldehyde molecules incorporated into the short chain polymer. The first paper focuses on n = 1, the second on n = 3, 4, and 5.
The authors describe the motivation for their papers as follows:
However, most existing process chain analyses rely on literature data on the separate process steps obtained from different sources11,17,20 or are based on simplified assumptions regarding product separation.15,16,18 Furthermore, detailed analyses of bottlenecks and points for improvements of a Power-to-OMEn process chain have not been presented. This is partly due to the fact that the thermodynamics of some of the mixtures involved are very challenging to model and so far have mostly been addressed with specialized models (e.g., ref 21) that are difficult to implement in commercial flowsheet simulators. Most existing detailed simulation studies,22,23 on the other hand, only consider single process steps and not the entire process chain. Therefore, the purpose of this two-part paper is to conduct a detailed analysis of Power-to-OMEn process chains based on existing process steps to identify the potential and bottlenecks of the established concepts and to provide model implementations that can serve as a starting point for the analysis and development of new process concepts using a commercial flowsheet simulator.

(R1)
While alternative synthesis pathways such as selective direct oxidation of methanol in the gas phase27 or reaction of methanol with H2 and CO2 through homogeneous catalysis28,29 have been suggested, these are at an earlier stage of development and are hence beyond the scope of this work. We thus consider a process chain consisting of three existing process steps for converting H2 and CO2 to OME1 via methanol and aqueous formaldehyde solution (FA(aq)) as shown in Figure 1.
Figure 1:
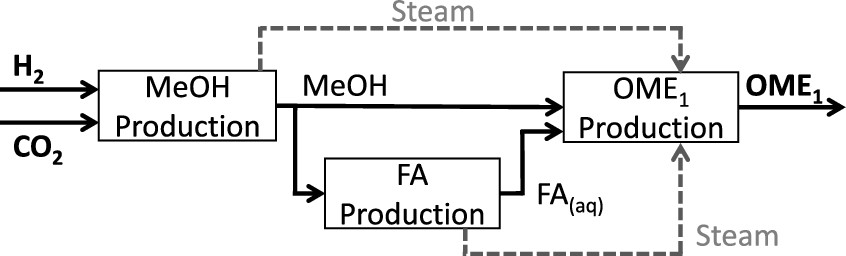
The caption:
Since the paper focuses on beginning with hydrogen and carbon dioxide it is now (thankfully) agnostic with respect to the source of primary energy; we are now free to consider thermodynamically superior and much more environmentally sustainable nuclear energy and case lose the wind and solar fantasy.
The authors give their process assumptions for methanol as a straight up hydrogenation process, shown in this diagram:
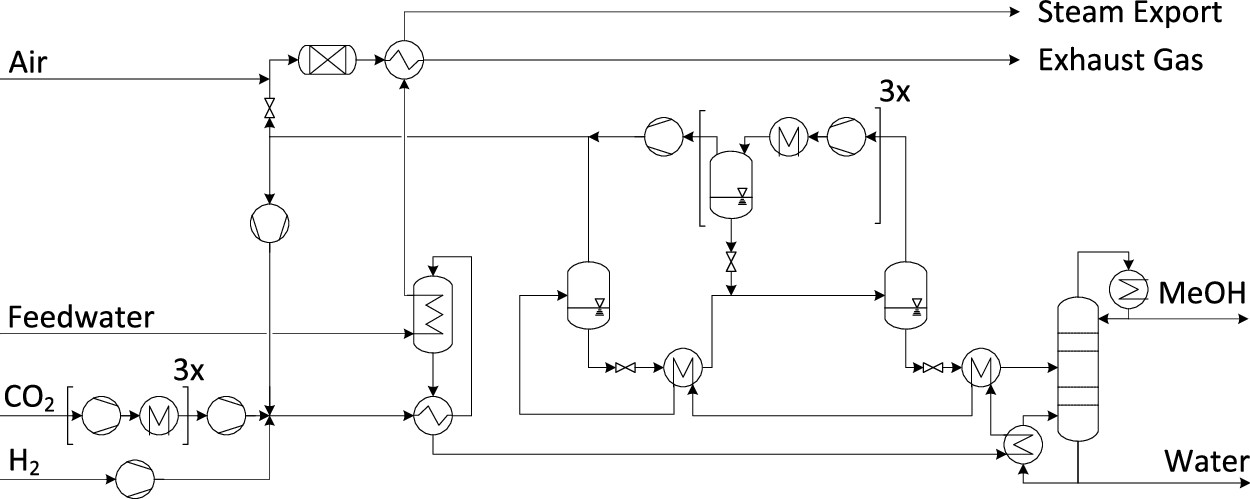
The caption:
There's no real mystery here; the scientific literature oozes with ways to make methanol from CO2 and hydrogen, although in reality, it is mostly, overwhelmingly, made by the partial oxidation of dangerous natural gas. They key to decarbonization is to make hydrogen from water and not dangerous natural gas (or worse, coal) and to make methanol (or a methanol equivalent like DME) from carbon dioxide and hydrogen and not dangerous natural gas (or worse, coal).
The authors propose to make formaldehyde by the partial oxidation of methanol.
They write:
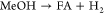
(R2)
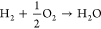
(R3)
Note that R2 and R3 are selected for ease of modeling (cf. section 3.2) because the actual partial oxidation reaction MeOH + O → FA + H O 1 2 2 2 is a linear combination of R2 and R3 and is hence not needed in a conversion-based model. Main side reactions are the formation of carbon monoxide (CO) and its oxidation to CO2
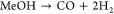
(R4)

(R5)
The reactor effluent is rapidly quenched and fed to a four-stage absorption column where formaldehyde is separated from the product gas using water as a solvent to yield an aqueous formaldehyde solution of 50 wt% strength. The off-gas contains significant amounts of H2 and CO and is hence burned in order to generate 5 bar steam in addition to that generated from the reactor effluent quenching.
Hydrogenating carbon dioxide to methanol and then re-oxidizing it to formaldehyde is a thermodynamically wasteful process from where I sit. I don't actually keep up all that much on the industrial chemistry of formaldehyde production, but undoubtedly it is made from dangerous natural gas in which methanol is an intermediate using a starting material that represents primary energy. I am aware of some electrochemical reductions of carbonate (aqueous carbon dioxide) but in general electrochemical production schemes are wasteful since electricity is never primary energy. In some cases the use of electrochemistry is justified, as in the production of aluminum, titanium and other metals, since it is effectively the only continuous process route. The low solubility of carbon dioxide in water however does not suggest that electrochemistry is a good idea. Undoubtedly there are partial hydrogenations, using mildly poisoned catalysts that would work as well.
By the way, formaldehyde that one encounters in biology labs featuring dissections, or in mortuary science is not actually "formaldehyde" but is a technical aqueous solution known as "formalin." The process described here is a "formalin" process, not a formaldehyde process, since the product is a solution. Pure formaldehyde is a gas. In formalin, there is actually only small amounts of free formaldehyde, since the gas reacts with water to give a number of acetal type polymers terminating, when not cyclic, in a "hemiacetal" in which one of the oxygens is capped with a hydrogen rather than a carbon.
Here, from a paper in Scientific Reports is a nice picture of this equilibrium:
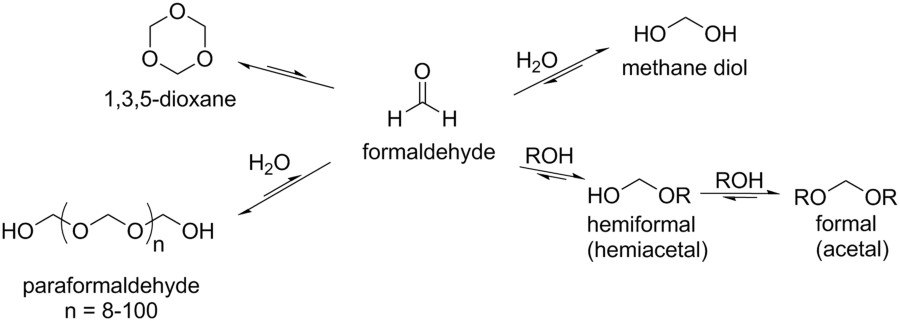
Note that the molecule above labeled 1,3,5 "dioxane" is labeled incorrectly in this diagram. It is 1,3,5 trioxane. 1,3,5 trioxane, a cyclic formaldehyde polymer, will be discussed below.
Be that all as it may, here is figure 3, on the production of formaldehyde by the re-oxidation of methanol produced from (in theory) hydrogen and carbon dioxide:
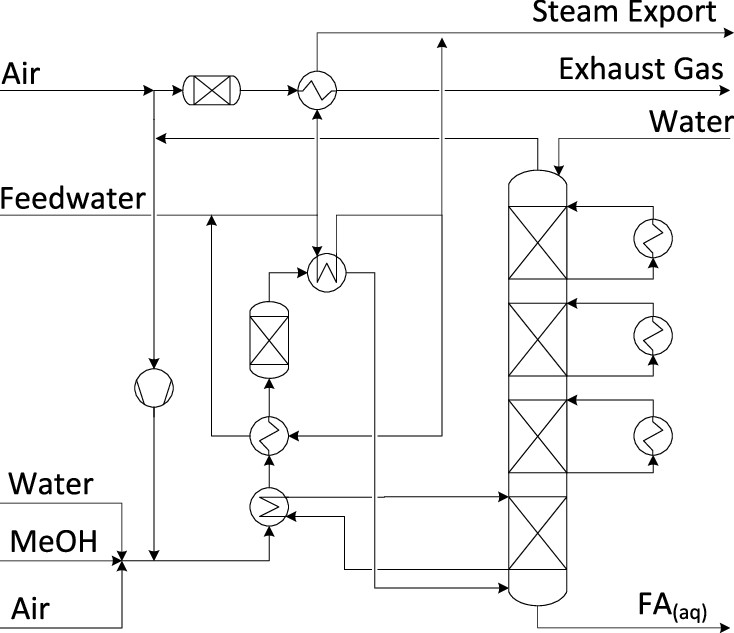
The caption:
When the formalin is combined with methanol to give methyal a difficulty arises inasmuch that methylal forms an azeotrope with methanol. An "azeotrope" is a situation wherein a mixture of two (or more) components boils at a temperature lower than the individual components themselves giving a vapor phase of a predefined composition; a famous example is ethanol and water, which distills at a temperature lower than either water or pure ethanol, giving a condensate that is 95% alcohol and 5% water, "190 proof." Solutions that form azeotropes cannot be isolated in their pure form by simple distillation, and usually one requires a chemical trick - to make "pure grain alcohol" the alcohol can, for instance, be distilled over calcium oxide (quicklime) which reacts with water to form calcium hydroxide (slaked lime) - or sometimes a physical trick with specialized multistage distillations.
Here the physical trick is to use a distillation system operating at two different pressures, a two stage distillation. Here is a diagram of the process. The following graphic shows the distillation apparatus the authors propose to break the azeotrope:
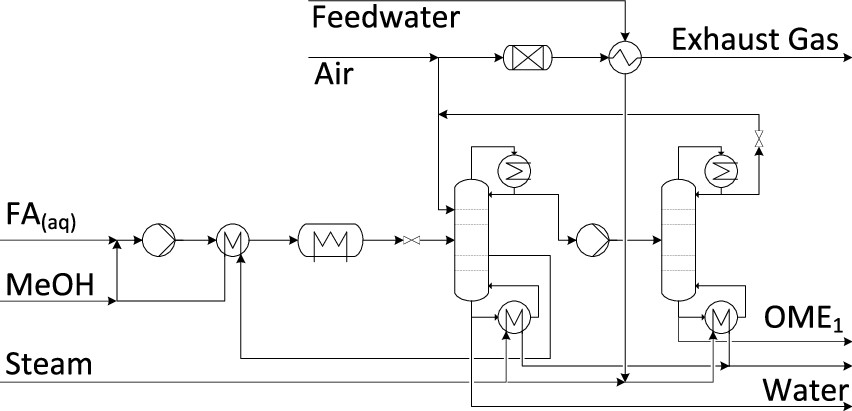
Note the energy intensive introduction of steam, a questionable approach for a system that is supposed to operate on solar and wind electricity.
Let me be clear on something: Any system that relies on diffuse and inconstant energy to drive a process of distillation of high boiling liquids will either involve huge areas - the natural system of evaporation and rain involves the entire planet - vast quantities of mass, poor thermodynamics, economic absurdity and environmental tragedy.
Distillation is a widely used technology and all commercial distillation devices must use energy at least having the energy/mass ratio of dangerous fossil fuels or electricity relying, in bulk of production, on devices depending on energy dense dangerous fossil fuels or - this is a great moral and environmental tragedy - to a lesser extent, nuclear energy. (The primary and largest use of distillation is, of course, the dangerous fossil fuel industry itself in oil refineries.) The extent to which electricity is used for this purpose represents a thermodynamically, and thus environmentally, questionably inefficient waste of energy.
All of this said, it is possible to construct systems where energy that would otherwise be waste can be used for the purpose of distillation of various commodities ranging from high boiling point materials to low boiling materials, rejecting smaller amounts of energy to the atmosphere and thus raising efficiency to very high levels, something I'll discuss near the end of this long and tiresome post. In the case of nuclear fuels, which can have temperatures internally of thousands of degrees, at least locally - the design of current nuclear reactors involves the dissipation of this heat rather than its direct use - the use of distillation can include things like liquid metals, either for the purpose of heat exchange or for purification, down to relatively low boiling commodities like methanol in an intricate network of heat exchangers.
Systems like this come under the general rubric of "process intensification," and the current issue, as of this writing, of Industrial & Engineering Chemistry Research, this one, Industrial & Engineering Chemistry Research, Volume 58, Issue 15 is a special issue devoted to just these issues.
Here, for amusement, is an equation from a paper out of UCLA entitled Process Intensification of Multipressure Reactive Distillation Networks Using Infinite Dimensional State-Space (IDEAS) (Flavio Eduardo da Cruz and Vasilios I. Manousiouthakis, Ind. Eng. Chem. Res., 2019, 58 (15), pp 5968–5983):

The point here is not to claim that I am even remotely competent to work with equations like this - I am not - but rather that there are, particularly in computational space, people who can, and who do. One can be sure in these times that there are people as well who can translate a set of equations such as these into relatively “idiot proof” commercial software that not only handles the iterations of summations, but also engages in machine learning to optimize empirical constants, such as those in sophisticated equations built on the Antoine equation. Although we are living in an age of the rising celebration of ignorance by aggressive and powerful ignoramuses, there are these islands of insight and reality still remaining in the scientific and engineering sphere much like Andre Gregory's description of Findhorn in the aesthetic and spiritual sphere. It is therefore beautiful that these scientists call their distillation network, the "Infinite Dimensional State Space" "IDEAS."
Ideas, and not ignorance, will save the world, if it can be saved.
Now, from the first paper under discussion here, some notes on the thermodynamics of this process after one has already isolated hydrogen and carbon dioxide, something that will not, with all due respect to the authors, be available from so called "renewable electricity."
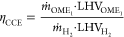
where LHVi denotes the lower heating value. It differs from the first-law efficiency in that it does not account for heat or work transfer. Instead, ηCCE measures how much fuel energy can be obtained from a given amount of H2, which is the main energy source and cost driver6 and hence likely to be a limiting factor in fuel production. To account for heat and work input in a consistent manner, we also consider the exergy efficiency for the conversion of H2 and CO2 to OME1 defined as
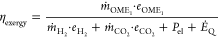
where ṁi denotes the mass flow rate of stream i, ei denotes the specific exergy, Pel denotes the electricity consumed by the process chain, and ĖQ denotes the sum of the exergy input associated with heat demand. The ambient temperature is set to 298 K. No distinction is made between exergy destruction and exergy losses to the environment (cf., e.g., Benjan et al.67), and for representation in the Sankey diagram, the sum of both is computed as the difference between the exergy of streams entering the process step and that of useful streams leaving the process steps.
The Sankey diagram is a graphic representation of the overall thermodynamic efficiency, again ignoring the huge thermodynamic penalties of procuring the hydrogen and carbon dioxide in the first place, penalties that dominate the commercial and environmental feasibility of the entire process.

Figure 5. Sankey diagram of the benchmark process chain based on the simulation results. The width of the arrows is proportional to the exergy of the respective streams.
To the author's credit, they allude to as much:
The bold, a genuflection to the reality of so called "renewable energy" as opposed to the endless mindless hype that resulted in the atmosphere surging at an unbelievable and terrifying rate, permanently past 400 ppm of the dangerous fossil fuel waste in the planetary atmosphere in November of 2015, to the current value of 414 ppm less than four years later, is mine. The fact, despite all the self-delusion commentary to the contrary, if not Trumpian scale outright lying, is that so called "renewable energy" is indelibly welded to and dependent on access to the very dangerous fossil fuels which are killing the future.
Figure 6:
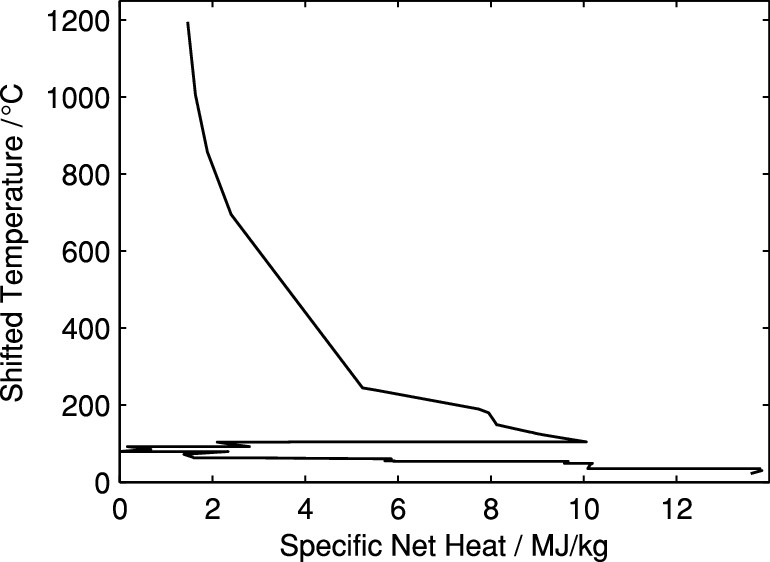
The caption:
The first sentence is pure nonsense. To beat the dying horse, we have just spent half a century and trillions of dollar proving that "renewable electricity" is decidedly not an option for climate change mitigation, since the period in which this irrational belief system has held sway, the opening decades of the 21st century, have been the opposite of "mitigation," that is they have marked an acceleration of the atmosphere's degradation. The attachment to the existing infrastructure is also questionable.
The rap on DME - my personal favorite carbon based fuel if it is prepared using nuclear energy as a primary energy source - as being unable to address this infrastructure is dubious as well. The truism that "those who say a thing cannot be done, should not interrupt those who are doing it," applies, at least to a limited extent. Volvo has been a leader in developing DME powered diesel engines, and apparently Volvo has acquired Mack Trucks. A test of ten Mack DME trucks is underway with the New York City Sanitation Department. This is an ideal place to test these trucks, since a potential source for DME is garbage, reformed at high temperatures either by water - or by carbon dioxide followed by water:
Mack Trucks and New York Launch Dimethyl Ether Fuel Test Existing diesel engines can run fine on DME if minor modifications are made, chiefly the chemistry of polymer seals, changes to the injectors, and modifications to fuel pumps. Volvo/Mack has demonstrated it can do these things. Indeed, in many areas - New Jersey is one - one can see trucks and buses that display prominently that they run on "clean" natural gas, even though there is nothing at all beyond pure marketing to suggest that natural gas is anything like "clean" other than when it is compared to even worse fuels, petroleum or coal.
The major problem with DME concerns its lubricity, a factor for which methane is clearly worse. I am not familiar with the lubricity of the members of the OMen systems described in these papers, but by analogy with PEG (polyethlyene glycol) with which I have considerable experience, these papers, the unrealistic sources of primary energy notwithstanding, intrigue me, since I would expect the OMen ethers to be very soluble in liquified DME, representing a potential additive that would work to increase engine and pump life.
From the next paragraph of the introduction:
"Mobility is supposed to be environmentally friendly..."
Well perhaps...
While, again, I have no use for the car CULTure I believe that certain kinds of vehicles should be available in a just and sustainable human culture, things like tractors, delivery trucks, ambulances, etc...
Given the availability of DME, and perhaps these OMEn ethers as recovered from "waste" energy I agree these fuels hold promise, which is why I value this paper, my criticisms notwithstanding. The fact that the authors note that currently the OMEn ethers are being produced in China from coal, it is clear that they can also be produced from biomass or, for that matter, trash, sewage sludge and perhaps even algae, both present as contaminants of eutrophied bodies of water where it would otherwise choke the oxygen supply killing the ecosystem (as in the modern day Mississippi Delta), or as created in constructed wetlands to recover phosphorous from sewer water. Thus the last sentence in the reproduced paragraph can be qualified as partially true, with the caveat that "renewable feedstock" is not created by the thermodynamically inefficient use of electricity.
The authors' graphic on their proposed OMen system:
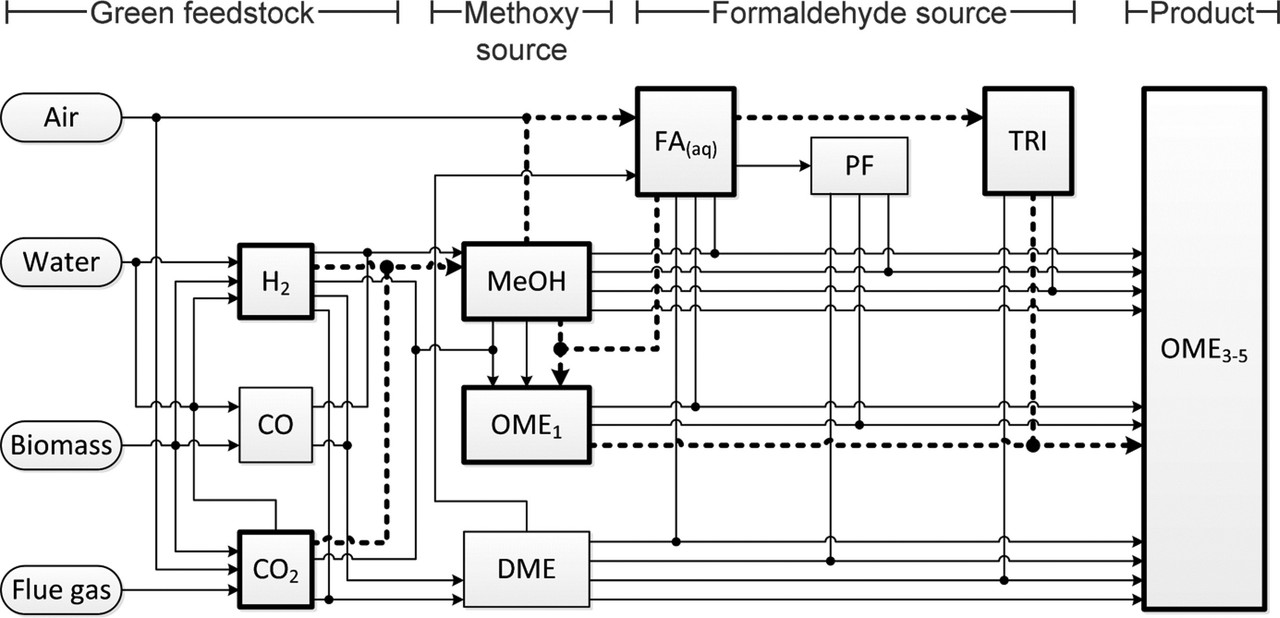
The caption:
There is in this paper considerable discussion of the "formaldehyde source" in terms of the chemical form in which the formaldehyde is delivered, that is in the nature of the "formaldehyde equivalent," the options being paraformaldehyde or 1,3,5 trioxane, shown in the graphic above in this post, in which the latter is incorrectly labeled "1,3,5 dioxane."
For the trioxane case, the following schematic is offered:
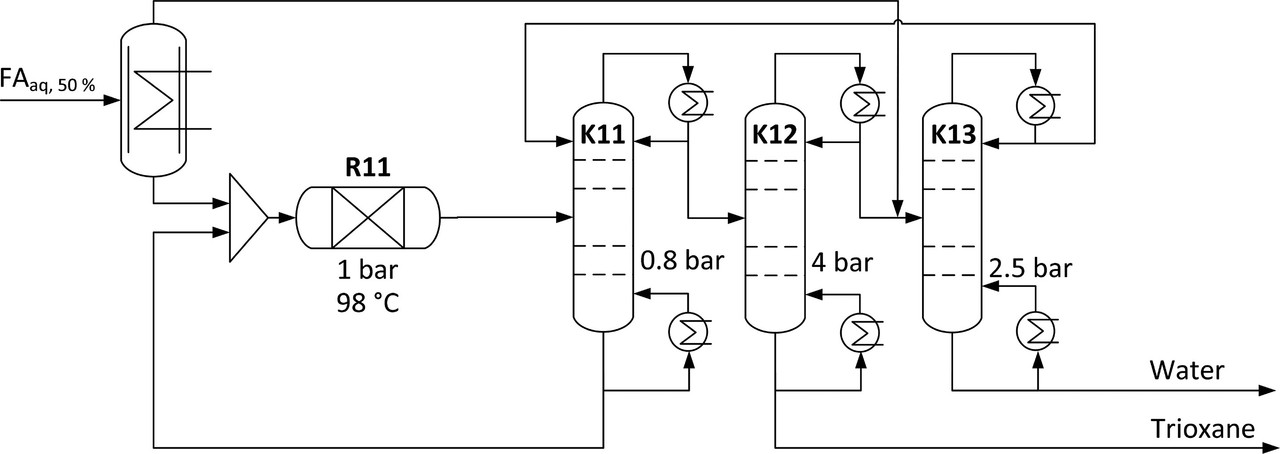
The caption:
Figure 2. Distillation-based trioxane production with three columns at different pressure levels, one falling film evaporator, and one reactor at the conditions given by Grützner.(49)
In a table in the text, the temperature of the R11 reactor is assumed to be 98 °C; the maximum temperature in the K-11 module is 93 °C; in the K-12 module, 165 °C; in the K-13 module, 127 °C. It is important to note, I think, whether or not the authors consider it, although clearly they do a lot to consider process intensification, that the primary source of this heat is supposed to be so called "renewable energy" which, to return to the main point, is supposed to be variable. Turning the heat on and off, this at high temperatures well above the boiling point of water, depending on the uncontrolled availability of energy. In this light, all other calculations need to be taken with a grain of salt, at least in my opinion.
Some text associated with the production of the final product is worth considering:
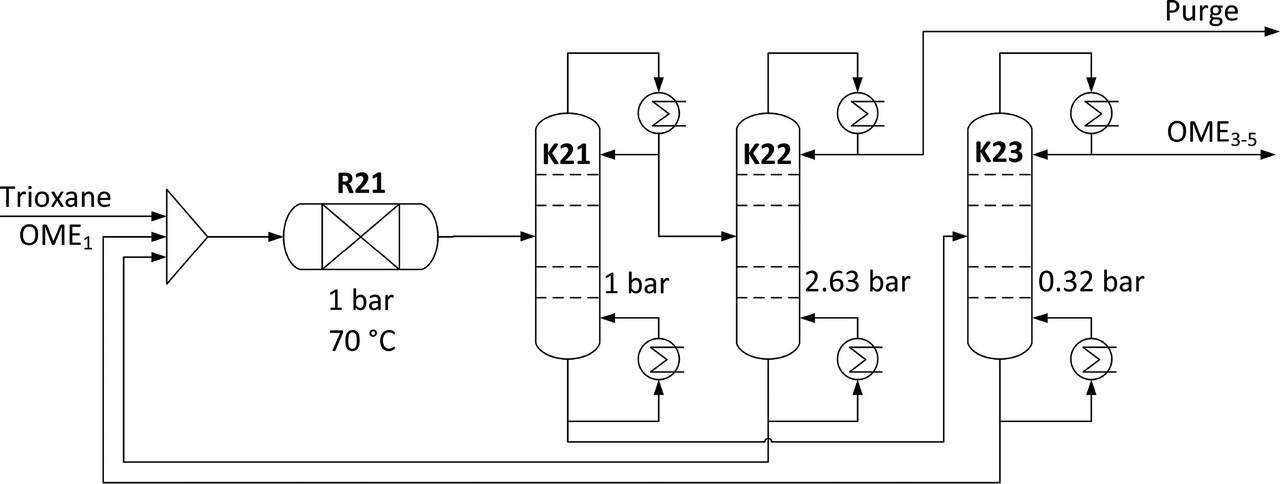
The caption:
According to the tables in the paper, R21 operates at 70 °C, K21 has a maximum temperature of 186 °C;K22 has a maximum temperature of 83 °C; K23 has a maximum temperature of 250 °C, temperatures again all to be provided by the use of variable so called "renewable energy."
It is useful to take a look at the mass flow Sankey diagram:

The caption:
As an environmentalist, I really don't like that "exhaust" feature from air, since this represents the air oxidation of methanol, a toxic alcohol, albeit one found commonly in automotive window washing fluid, to produce formaldehyde. Personally I have a NIMBY reaction to living anywhere near a plant doing this, at least whenever the wind is blowing and/or the sun is shining brightly.
The authors write of the chemical reaction (mass) efficiency the following:

Concerning the amount of CO2 required for producing 1 kg of OME3−5, an overall carbon-based yield of 87% is reached.
As an aside, given what I think is the only form of energy that is environmentally acceptable and sustainable, nuclear energy, popular madness not withstanding, that the energy content of a kg of plutonium-239 is 80.2 million MJ, and thus equivalent of 419 million kg of OME3-5. A kg of plutonium could fit in a small coffee cup. Somehow, incredibly, there are a large number of people on this planet who think that dealing with the so called "waste" from quantities that can fit in a coffee cup is more problematic than dealing with the waste of handing millions of tons of waste, which is exactly what OME3-5 will become when it leaves a tail pipe.
No wonder we're seeing more than 35 billion tons of carbon dioxide waste indiscriminately and thoughtlessly dumped every year into the planetary atmosphere. We cannot think clearly.
It is very useful to look at the author's assumptions, some of which are likely to be more valid that others on a practical level.
Let me begin with a description of their assumption of how electrolytic hydrogen is made and whence the carbon dioxide is assumed to come:
Almost none of the world's industrial hydrogen as of 2019 is produced by electrolysis, and of that which is, very little is produced using PEM systems, even though commercial examples of PEM systems are known. The overwhelming bulk of the tiny fraction of hydrogen produced by electrolysis is produced in alkaline hydrolysis units, which I'll briefly discuss below in terms of their lifetimes and efficiency. It is worth noting from a purely environmental standpoint that PEM systems use Nafion proton exchange membranes, which are highly fluorinated polymers likely to have extremely persistent lifetimes and, when they do degrade, release highly persistent fluorinated gases. Who cares? I'll return to this consideration a little later, but for now, I'd like to consider the portion of the text that I, and not the authors have put in bold.
There are three implied cases here: One, the most telling is the statement about flue gas, indicated, once again that most"renewables will save us" fantasies, none of which have had any effect on the on going degradation of the planetary atmosphere, depend on the assumption of the continued access to dangerous fossil fuels. The use of flue gas for a carbon dioxide source, particularly for a fuel whose combustion gases will be dumped indiscriminately into the planetary atmosphere through tail pipes, is not acceptable. So there's that.
I personally believe that it will be necessary, given the indifference of our generation and all preceding generations to the dumping of carbon dioxide and entropy into the planetary atmosphere that future generations will be required and their and not our expense, to actively remove carbon dioxide from the air. But the handwaving in this context, a glib assumption that this can be done without enormous, absolutely enormous energy requirements is, frankly, obscene. As for biogas, well, I note that all the straw in China, all of it, amounts to less than one billion tons, this on a planet where the annual dumping of the dangerous fossil fuel waste carbon dioxide is on the order of 35 billion tons. Biogas will always be trivial on scale.
If you think this energy requirements to accomplish air capture can be provided by wind and solar energy, I really can't help you, because you are so divorced from reality as to boggle the imagination. So in this context, air capture is a non-starter. The evocation of it is pure hand waving.
The authors optimistically cite process intensification, as described above, for heat flows.
OK, maybe. I personally believe that process intensification is a very good idea, and most of my own musings about what we must do if we ever become serious - which in my view we aren't - involve process intensification. I note that whether process intensification involves clean and sustainable nuclear energy or less clean and less sustainable so called "renewable energy," this type of infrastructure does not really dominate world processing infrastructure. There are huge considerations in materials, chemical, and thermal engineering involved, less onerous, I believe, in the nuclear case than in the so called "renewable energy" case.
In either case, such infrastructure does not exist currently.
Sundry other considerations, compression, expansion, etc. They are neatly summed up in the graphic below:
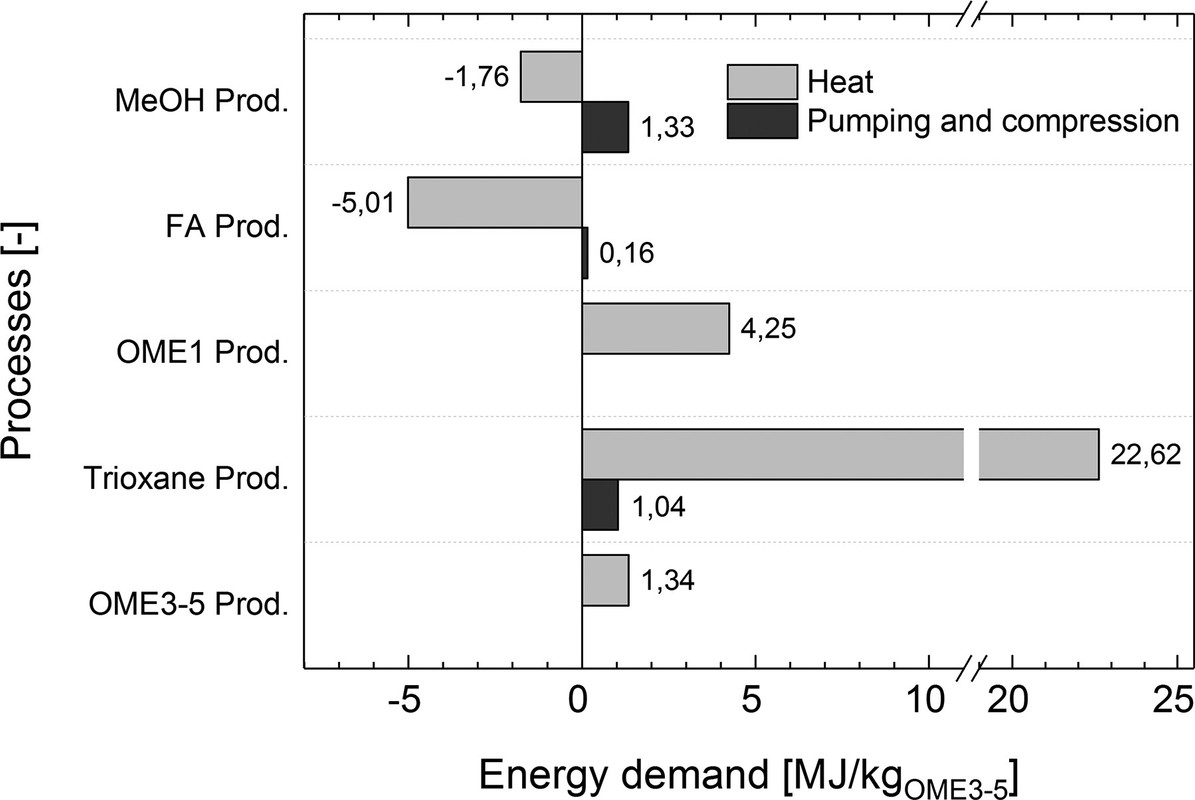
The following graphic refers to a process detail concerning the degree to which formaldehyde is converted to its cyclic polymer trixoane.
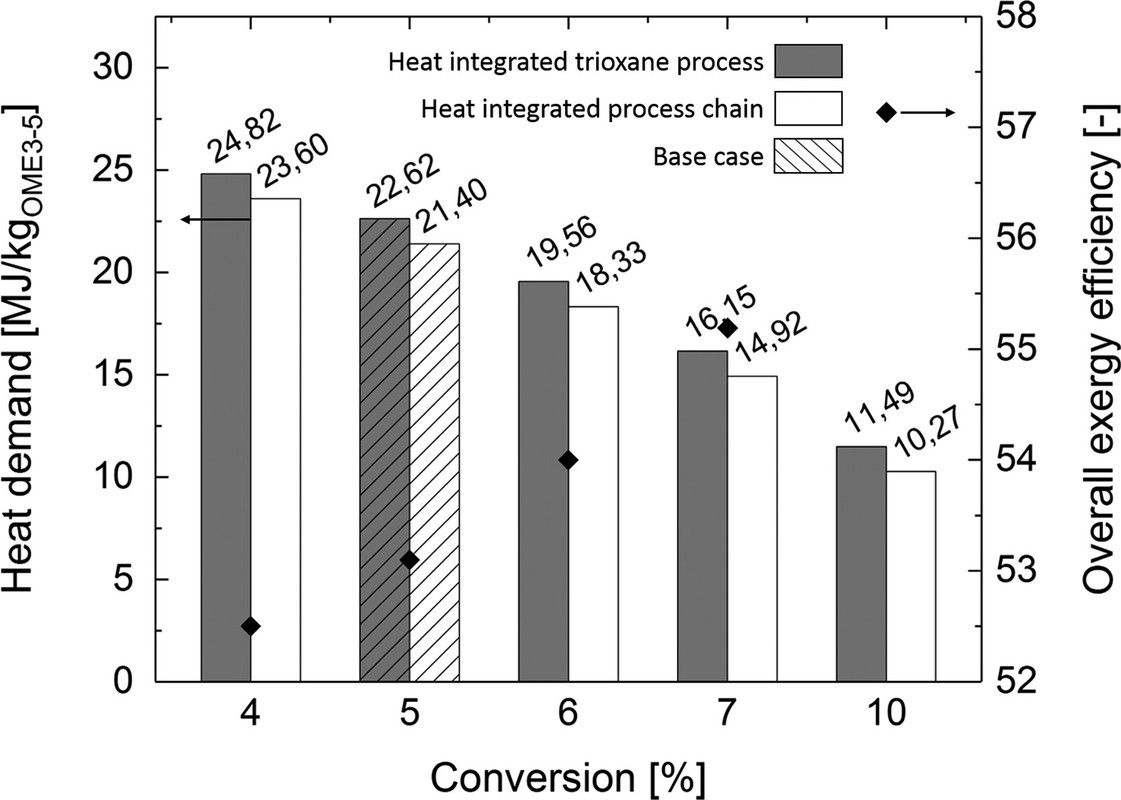
The caption:
It doesn't matter much. What does matter is the overall energy efficiency of the process, described in the following Sankey diagram, which requires some reference to the text, to avoid it being too misleading:
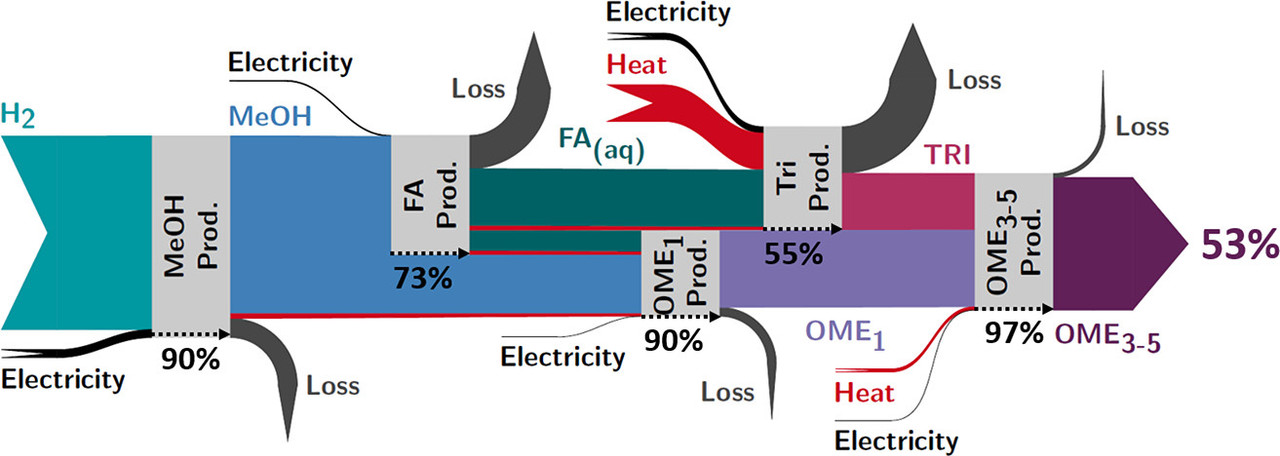
The caption:
The 53% figure is, of course, consistent with the case I often make whenever I hear dreamy bullshit about how easy it will be to overcome the fact that so called "renewable energy" is intermittent. I contend quite the opposite, that it is not easy, nor, given the unacceptable environmental impact that so called "renewable energy" has while remaining trivial, will it make so called "renewable energy" better. The opposite is true. It will make an already unacceptable environmental impact worse.
However, I know from years of study that we have not only dumped the dangerous fossil fuel waste carbon dioxide on all future generations as an expression of our contempt for them; we have also dumped entropy. While the quantities of carbon dioxide are tremendous, the concentration is dilute. It will take thermodynamic work to concentrate it.
Thus one may ask, is it really the case that the efficiency here is 53% as the graphic implies?
Let's look at the following text:
Before turning to the sentence I have put in bold in the above excerpt, let me say this: There are very few commercial hydrogen electrolyzers that operate at 80% efficiency. This paper lists the top 15 commercially available units and calculates their efficiency:
Hydrogen Production From Water Electrolysis: Current Status and Future Trends (Proceedings of the IEEE Vol. 100, No. 2, February 2012, 410-426). Only three of them demonstrate efficiencies this high; two are alkaline bipolar electrolyzers, one is a PEM unit. The others listed all range from below 50% efficiency to roughly 75% efficiency. Moreover, this paper, Applications of power to gas technologies in emerging electrical systems (Andrea Mazza⁎, Ettore Bompard, Gianfranco Chicco Renewable and Sustainable Energy Reviews Volume 92, September 2018, Pages 794-806) lists some factors that put lie to the claim that alkaline electrolyzers are really suitable in any fashion whatsoever to work with variable and intermittent energy. To wit:
However, some drawbacks still have to be overcome [21,23] concerning:
• The minimum load. It is not possible to operate the electrolyser over the 0÷100% Pn range: the minimum load usually falls within the 10÷40% Pn range [37], even though a case with 5% Pn is reported in [38]. This means that if the input is lower than these limits, the electrolyser has to be switched off.
• Transient operation is possible with this technology, but some problems can arise: in fact, the typical response time is seconds or minutes, but according to [37], these are not physical limits, but only design limits that depend on the absence of a fast response by the customers.
• The relatively long cold start time (10 min to h), which mainly dependson the purity of the gas [37].
• The long restarting time after shutdown. This is an important drawback, because the electrolyser takes 30–60 min before it can be switched on again (due to purging operations with nitrogen)
In other words, some of the energy said to be available for storage will necessarily be wasted just waiting for the time for the reaction to start, low load variations can effect the efficiency and performance.
PEM electrolyzers perform better, but cost twice as much money, often require elevated temperatures to operate, and - important to any consideration of scale - billions of tons of oil are burned each year - they involve a lot of highly fluorinated polymers, this in a time where fluorinated carbon compounds are emerging as major intractable persistent pollutants. The lifetime of PEM electrolyzers is thought to be on the order of 5 to 20 years before they require replacement.
And now let's turn to the text from the OME3-5 paper which I have put in bold, the bold being this remark:
Recall the remarks made earlier in the paper, to wit:
Of the three sources listed, one is likely to be available only on a small scale (biogas plants), one (flue gas) a confession that the use of this so called "renewable energy" like the rest of this crap assumes and depends on the availability of dangerous fossil fuel, and the last, air, is extremely dilute, 0.0414% (414 ppm) as opposed to 13%. The extra dilution means the requirement for extra energy to overcome the entropy of mixing, huge amounts of energy.
(I've spent many years pondering this problem. I believe air capture is on the edge of technically possible, but the engineering and energy challenge is vast, and no, no one is going to do this with solar and wind, except in wholly delusional dreams.)
For a nice overview of the thermodynamics of air capture of carbon dioxide, one may check out the widely cited paper by House, Economic and energetic analysis of capturing CO2 from ambient air (House et al PNAS December 20, 2011 108 (51) 20428-20433). The paper has been the subject of some criticism which I think (or I hope) may be justified.
Speaking of justification however, we are probably justified in assuming that "flue gas" will be available for a long time, since the common pop perception is that so called "renewable energy" is an effective means of addressing it persists. This is an affectation that has nothing to do with reality of course: So called "renewable energy" has not replaced, is not replacing and will not replace dangerous fossil fuels.
One of the world leaders authorites on and leading researcher on direct air capture (DAC) of carbon dioxide is Doctor Christopher Jones of Georgia Tech. A few years back he wrote a nice reviews of the subject of air capture of carbon dioxide: Direct Capture of CO2 from Ambient Air (Jones et al, Chem. Rev., 2016, 116 (19), pp 11840–11876). In it he refers, in detailing the difference in requirements for capturing carbon dioxide from flue gases and direct air capture and gives us some information about the concentration of carbon dioxide in these various streams. To wit:
The bold and the italics are mine.
In other words, to assume that one can obtain as overall thermodynamic efficiency as high as 38% - not the 53% graphically represented in the Sankey diagram - one must also assume that one will still be burning coal to obtain the carbon dioxide to make motor fuels from solar and wind energy.
Now that puts a slightly different spin on the whole affair, does it not?
Later on in the review Jones writes:
Reference 215 is this very interesting paper: Importance of Ambient O2 for Electrochemical Enrichment of Atmospheric CO2 (Chuan Wang†, Hong Liu*†, Xiangzhong Li‡, and Linze Zheng# Ind. Eng. Chem. Res., 2013, 52 (7), pp 2470–2476) with which I was unfamiliar until just now, even though it was in my files.
Reference 216 is the House paper to which I referred above.
By reference to the text of the House paper one can see that the 400 kJ/mol figure was not actually a target for active air capture of carbon dioxide but rather a "break even" point for getting electricity from dangerous natural gas, on which, increasingly, 8 years after House's publication, the world is increasingly investing, albeit with lots of delusional horseshit being thrown around about how wonderful so called "renewable energy" is. To wit:
The bold is mine.
Well then. OK.
The amount of plutonium that contains enough energy to remove a mole of carbon dioxide from the atmosphere, at 400 MJ/mol is about 5 milligrams, an amount so small as to be near the limit, maybe of what one can see with the naked eye, if one has excellent vision. It might require a microscope; I don't know; plutonium metal in all its phases is pretty dense.
A mole of carbon dioxide is 44.01 grams, meaning that there are 22.7 moles in a kg of carbon dioxide, corresponding to roughly 9.1 MJ/kg to capture carbon dioxide from the air! (There are better approaches, I think, to removing carbon dioxide from air via seawater, but that’s an entirely different topic.)
Be that as it may as I often point out, coal has been the fastest growing source of energy in the 21st century. It's not going anywhere, even if the United States is shutting coal plants and replacing them with gas plants; the US is also on a criminal path of shutting nuclear plants and replacing them with gas plants, the crime being against humanity, against all future generations.
Let's be generous, even if too much generosity is, as such, a crime against humanity, and ask how much must the solar and wind industry grow in order to replace, as suggested in this paper, all the world's petroleum with solar and wind energy if we blithely assume we will still be burning coal and having 13% carbon dioxide flue gas after deciding to build a huge solar and wind infrastructure to support a huge infrastructure to make chemical plants to make the OME3-5 fuel.
How much solar and wind energy would be required?
Whenever I post reference to the 2018 Edition of the World Energy Outlook Table 1.1 Page 38 to show exactly how useless, after half a century of cheering, so called "renewable energy" is I write the following:
Let me not do that now. MTOE is the abbreviation for "million tons oil equivalent" and it "assumes" a "standard" form of oil even though in real life, oil's energy content depends heavily on its geological/geographical source. The MTOE figure for oil consumption in 2018 is reported in table 1.1, Page 38 as 4435 million tons oil equivalent. That means that in 2017 the world consumed roughly 4.4 billion tons of oil.
Here, from the paper, is the accounting for the efficiency of the conversion process from carbon dioxide to OMe1-3 motor fuel:
Now of course, if one obtains the carbon dioxide from the flue gas of a coal plant, recovers it using so called “renewable energy” to make motor fuel for a car– not likely but let’s play pretend – it should be obvious to anyone with an ounce of sense that it still ends up in the atmosphere and represents a dangerous fossil fuel converted into the dangerous fossil fuel waste carbon dioxide. But suppose – again not likely but let’s play pretend – that the carbon dioxide is recovered from air. What would be the efficiency then?
One can calculate this from the numbers provided in the previous excerpt and from the earlier remark in the paper that the OME3-5 has an energy density (Lower Heating Value) of 19.16 MJ/kg. (We use the lower heating value because none of the work in an automotive engine results from the latent heat of condensation, i.e. the gases emerge from the pistons in the vapor phase and at elevated temperatures.) By reference to the mass based Sankey diagram above we can see the “stoichiometry” of the reaction, i.e. that 0.25 kg of hydrogen reacts with 1.83 kg of carbon dioxide to yield 1 kg of OME3-5. At the 383 kJ/mol reported in the Wang paper for their lab scale device, should it be scalable to industrial use, we can determine from the fact that, as above, a kg of carbon dioxide is 22.72 moles that a kg of air captured CO2 under these conditions would represent an investment of 8.7 MJ/kg at 383 (as opposed to 400) kg/mol . We multiply this by 1.83, add it to the claimed figure for electrolytic hydrogen (180 MJ/kg) times 0.25 and the figures for process heat, generously assuming the figures for the process intensified heat flows (17.94 MJ/kg OME3-5 produced). The sum of these figures so calculated is 78.9 MJ/kg
We thus find that the most optimistic case for carbon dioxide from air capture converted into OME3-5 gives a result of 24.2% overall energy process efficiency. If we were to replace all the oil consumed in 2017 according to the World Energy Outlook, 4.4 billion tons (4.4 trillion kg), we thus calculate that the total energy required to make 4.4 billion tons of OME3-5 would be 347 exajoules. Currently, again, after half a century of cheering, all of the solar, wind, geothermal, and tidal energy combined, as of 2017 was producing 10.63 exajoules. Thus, in the “percent talk” so favored by advocates of so called “renewable energy” the scale up would “only” be 3,264%, this only for motor fuels for our beloved cars and trucks, not for electricity.
Actually though this is a low estimate.
Here is a calculator for determining the lower heating value of various fuels: Hydrogen Tools Lower and Higher Heating Values of Fuels
Here are the figures in MJ/kg that I have obtained using it:
US Standard Diesel: 42.78 MJ/kg
Crude Oil: 42.68 MJ/kg
Fischer-Tropsch (FT) Diesel: 42.45 MJ/kg
Dimethyl Ether (My preferred fuel): 28.87 MJ/kg
All of these fuels have higher lower heating values than OME3-5. (Methanol comes in at 20.09 MJ/kg.)
Thus at 19.16 MJ/kg OME-3-5 has only, in percent talk, 44.9% of the energy value of crude oil. This means to displace petroleum, we would actually need 4.4 billion tons/0.449 = 9.8 billion tons of OME3-5. The energy required to make it from solar and wind would thus be 727 exajoules, greater than world energy consumption for all purposes in 2017 (584.98 exajoules), all from solar and wind, requiring a scale up, just for motor fuels, of "only" 7,272% in "percent talk."
No problem, I'm sure, given that solar and wind are purely magical.
It is worth considering how much steel and how much aluminum and how much copper would be involved in producing 727 exajoules of so called “renewable energy.” A commentary in Nature Geoscience a few years back discussed precisely this issue: Metals for a low-carbon society (Olivier Vidal, Bruno Goffé & Nicholas Arndt, Nature Geoscience volume 6, pages 894–896 (2013)) To wit:
I have put in bold the energy required as opposed to the peak power figures traditionally utilized in the big lies attached to so called "renewable energy's" alleged growth, growth that in units of energy is absurdly trivial given all the noise made.
12,000 TWh is 43.2 exajoules. By simple math 727 exajoules is 16.8 times larger, or in "percent talk" 1,680% more than what what the WWF predicted for wind and solar in "by 2050" talk.
The translates into 53 billion tons of steel, 5.2 billion tons of aluminum, and 673 million tons of copper. The production of steel involves coal, the current process for the production of aluminum utilizes petroleum coke electrodes (which are oxidized), and copper involves intense heat and the release of copious quantities of sulfur oxides.
Renewable? Really? Whither the ores for this pixilated process?
In the current issue of Nature an editorial writer deplores the fact that scientists in general do not publish negative results, experiments that fail. Rein in the four horsemen of irreproducibility
Although her remarks are geared to social science studies, they are relevant to other studies and bear reflection. She writes:
That has begun to change for two reasons. First, clinicians have realized that publication bias harms patients. If there are 20 studies of a drug and only one shows a benefit, but that is the one that is published, we get a distorted view of drug efficacy. Second, the growing use of meta-analyses, which combine results across studies, has started to make clear that the tendency not to publish negative results gives misleading impressions.
I certainly agree that if we made a habit of publishing negative results, a lot of effort, time and money could be saved; but of course, many of us are guided by belief and will not read or accept negative results. For instance, there are a whole set of Americans, including the idiot who nominally leads this country who refuse to believe that carbon dioxide is killing the planet. For another example there are a whole set of Americans and other people around the world who believe that so called “renewable energy” is a viable approach to addressing the carbon dioxide crisis. The negative result that carbon dioxide is destabilizing the weather is readily observable, as is the negative result that the expenditure of trillions of dollars on so called “renewable energy” has done absolutely nothing to slow the accumulation of carbon dioxide or protect future generations of human beings and other life forms.
The result of both beliefs stated in the last sentence is the same. The planet is being destroyed, not because it has to be, but because in both cases seeing is not believing.
To the credit of the authors of the study of the OME1 and OME3-5 papers under consideration, they draw a negative conclusion from their work, i.e. that these fuels are almost certainly not worth pursuing:
The reference for the efficiency of "other e-fuels" by the way is open sourced. It is here:
E-Fuels−Mehr als eine Option; DECHEMA, 2017.
It's in German, but even if you don't speak German, it is worth at least looking at table 1. The numbers for the various devices there are in general, fairly reasonable in my experience, at least within 10% in most cases, but they need to be taken with a grain of salt. For example, the German word for "battery" is very much like the English word, and shows 95% energy efficiency, which is true, as the first graphic I produced in this post suggests, for some batteries, but hardly all of them, and the manufacturing cost is ignored. The reported efficiency (Wirkungsgrad) in this table for an electric motor - Elektroantrieb - is 90%. This suggests that an electric car, which uses both devices has an efficiency of 90% * 95% = 85.5%. However, as discussed near the beginning of this post, the electricity to charge the battery is not primary energy, but rather is a form of energy transformed from primary energy. Almost all of the world's electricity is still produced in power plants running on dangerous fossil fuels, with a Carnot efficiency of 33% - Combined cycle gas plants are higher, but still generally less than 50% efficient. Thus most electric cars have an overall energy efficiency of 85.5% * 33% = 28.2% efficiency which is lower, according to this table than the efficiency of an internal combustion engine, which is reportedly 30%. (The beautiful compound German word for internal combustion engine is Verbrennungskraftmaschine.)
I recently went to pick up my son his university for a weekend visit for the Easter Holiday, but he wasn't quite ready to leave when we got together. He wanted to complete an assignment due at midnight before going home so I suggested we hang out in the library. I left him to his work and wandered around the stacks, pulled a few books off the shelves that looked like a fun read. One of the books I pulled off the shelf in paper form was this one: Understanding non-equilibrium thermodynamics (electronic resource) : foundations, applications, frontiers / G. Lebon, D. Jou, J. Cases-Vázquez.
It is a good idea to look at books about subjects about which one thinks one already knows because most often there will be something that one doesn't know therein and thus one can deepen one's knowledge; and then there is the joy of seeing something beautifully and clearly written about a complex and somewhat popularly arcane topic. The first chapter of this book, a description of the history of thermodynamics, fits the category of "beautifully and clearly written," because it elegantly describes the mystery of thermodynamics, particularly on the description of what entropy is and how people came to understand both its existence and its implications. Entropy is not a state function for which one can pull out a device an measure directly, as one can do with temperature. (It included the interesting and compelling statement that none of what most people understand as equilibrium thermodynamics should actually be called thermostatics.)
The discovery of the concept of entropy – and the concept of energy itself - helped explicate the fact that thermodynamics is very much a function of path. In general, any thermodynamic process that proceeds via a path through electricity is poor. There may be chemical exceptions to this in some cases, for example the reduction of aluminum and titanium to their respective metals, or in issues involving morphology as in nanostructured coatings, but in the case where energy storage is the intrinsic goal this is true.
This brings me what I believe to be the only sustainable and environmentally acceptable form of energy, nuclear energy.
Developments in materials science have made it possible to increase the thermodynamic efficiency of dangerous natural gas plants from roughly 33%, the average value for a pure thermal power plant, to 50% or more in combined cycle plants which feature both Brayton cycles - like jet engines - and Rankine (normal thermal) steam cycles. In these plants, the exhaust heat from the high temperature Brayton cycle is used to boil water to drive a steam turbine. Although dangerous natural gas will never be an acceptable or safe fuel, this approach makes gas plants less odious since it converts more chemical energy into usable electrical energy than would otherwise be the case.
Almost all of the world's nuclear plants are dedicated to making electricity, and almost all of them likewise - although there are some exceptions - are Rankine cycle steam plants. (The Brayton cycle nuclear plants historically faced, during the golden age of nuclear plant construction, materials science limitations on their operations because of the high temperatures involved.)
Thus most of the primary energy in a nuclear plant is wasted energy; and in fact, although overall nuclear plants are responsible for almost no atmospheric pollution, in general, their thermal pollution is slightly higher than thermal pollution released by coal, gas, biomass and petroleum fueled power plants: The internal temperatures of nuclear fuels can be thousands of degrees Celsius in localized spots, and this heat must be removed to avoid Fukushima, Chernobyl, or Three Mile Island events, which despite much mindless selective attention directed at them, have had trivial environmental effects when compared to the growing use of dangerous fossil fuels.
There is no intrinsic reason connected with physics that nuclear energy plants need have the unacceptably low 33% thermal efficiency; as is the case with hybrid cars, much of the waste energy can be captured and put to use. This is especially true because of significant advances in materials science since the golden age of nuclear power plant construction, many of which derived from the aerospace industry where dealing with high temperatures was and is a necessary component. Early nuclear plants were built using technology from the 1940’s and 1950’s which was well before Yuri Gagarin and John Glenn orbited the earth. The golden age of nuclear power plant construction ended shortly after Eugene Cernan returned from the moon. Advances in thermally resilient materials continued after Cernan’s return of course, and this is why we can build combined cycle power plants now but couldn’t as late as the 1970’s. I say all this because the key to capturing the waste energy of nuclear reactors is raising the temperature at which they operate, designing them to operate normally at the temperatures which destroyed the Chernobyl and Fukushima reactors. Any refusal on your part to consider this possibility as being possible will not affect me in the slightest other than to make me aware that you know very little about either nuclear engineering or materials science.
Nuclear plants are capable of being utilized in a combined Brayton/Rankine system similar to dangerous natural gas plants with various kinds of gas coolants - my personal favorite Brayton working fluid would be the carbon dioxide used in the Western World's first commercial nuclear plant, Calder Hall, which operated almost half a century - without the noxious climate effects of dangerous natural gas.
But this would only address electricity, and not the manufacture of chemical fuels. In the chemistry case, we would have thermal energy converted directly to chemical energy (without an electricity intermediate) at very high temperatures, and when cooling these chemical fuels, it is possible to generate electricity (either Brayton, Rankine or both) while cooling the generated fuels as a side product. Such a system might well convert nuclear energy with very high thermal efficiencies, conceivably to efficiencies in excess of 70% as compared to 33% for the pure (Rankine) electricity generation.
I have written here and elsewhere on the internet, about thermochemical cycles, probably most recently here:
Cerium Requirements to Split One Billion Tons of Carbon Dioxide, the Nuclear v Solar Thermal cases. In this case the gas split was carbon dioxide; in many other cases the split gas would be steam. (The destruction of the Fukushima reactors was partially connected with metal catalyzed thermochemical water splitting to generate hydrogen, although a zirconium based controlled water splitting scheme would almost certainly not be effective.)
It doesn't matter which gas is split actually, hydrogen is always accessible from carbon monoxide; in fact 99% of the world's commercial hydrogen is made by the water gas reaction, which utilizes dangerous natural gas that is partially oxidized to carbon monoxide, and mixed with steam to produce hydrogen and carbon dioxide.
(One of the processes listed in the table in German to which I linked above is the “RWGS + CO2” process, the reverse water gas reaction, which is a neat way of splitting carbon dioxide to generate carbon monoxide and oxygen by consuming hydrogen. It’s been considered as a possible tool in human space flight.)
Of course, one still needs a source of carbon dioxide in order to split it.
A supercritical fluid is a fluid that is neither a gas or a liquid but has properties of both. (It is distinguished by the disappearance of heats of vaporization in its heating curves.) The supercritical point of water is 373.946 °C and the supercritical pressure is 22,060 kPa, just under 218 times the pressure of the atmosphere. When seawater is raised above this temperature at pressures higher than 218 atmospheres it separates into two supercritical phases, one that is essentially pure water and the other that contains nearly all of the salts. As the temperature rises beyond the supercritical point, all of the organic materials ultimately reduce water to form hydrogen and carbon dioxide.
One of the things that sort of bothers me as an environmentalist is the destruction of river delta ecosystems, either by eliminating water flows from the river (as in the Colorado River) or by loading chemicals into the water that kill all the life at their mouths (as in the Mississippi River.) The chemicals that destroyed the Mississippi River Delta are actually chemicals that are essential to life, fixed nitrogen and phosphorous. This, of course, is eutrophication, the loading of so many nutrients into water flows that the growth of algae explodes in them. The layers of algae become so thick that the lower levels are cut off from light and die and sink to the bottom of the body of water - in this case the Mississippi estuary - where they rot. The bacteria that rot them are aerobic and they greatly lower the oxygen levels in the water, leading to the death of species, crabs, oysters, shrimp, fish, etc that need higher levels of oxygen to survive than the bacteria do. The dead zone at the mouth of the Mississippi River, last I looked, was about the size of the State of New Jersey.
One of my favorite types of questions to hear - and one I personally employed more often when I was young and stupid - begins with the words, "Why don't 'they' just..."
No one ever stops to consider that 'they,' whoever 'they' are, may have thought about the same thing, but know excellent reasons why it won't work, or have tried it and showed why it won't work or simply lack the money to try it out.
In droughts in California and in New Jersey in my experience this is often the preface to the question "Why don't 'they' just desalinate water." In fact in many places in the world 'they' do just that, albeit at high cost, the desalination of water requires energy, irrespective of whether it involves ordinary distillation, flash distillation, RO membranes, ion selective membranes, whatever...
I've had a "why don't 'they'..." question about the Mississippi Delta floating around in my head, but more or less assumed that there was probably a good reason why 'they' don't utilize the Delta's algae as a source of carbon and phosphorous recovery. And while it seemed like a pretty good strategy to me, I certainly realize that as a species we are too invested in satisfying the stupid and the irrationally fearful to recognize a practical way to do this other than write long press releases how "by 2100," when the person writing the release will be dead, we'll all live in a "renewable energy" nirvana where we'll have plenty of energy from the sun and the wind do do whatever we want.
As it happens, while having this "why don't 'they' question bouncing around in my head for a number of years without looking into it, since I recognize that the irrational hatred of nuclear energy is killing ecosystems at an increasing rate and no one give's a rat's ass about it, I noticed just last week that there is a 'they' who are actively considering this approach.
The paper is here: Characteristics of Cyanobacterial Biomass Gasification in Sub- and Supercritical Water (Huiwen Zhang* , Xiaoman Zhang, Lei Ding, Jiangya Ma, and Yanli Kong,* Energy Fuels, 2019, 33 (4), pp 3239–3247)
From the text:
The experiment:
The temperatures involved were as high as 500°C, supercritical water temperatures. The operative thing here is that the biomass did not need to be dried. At these temperatures the constituents of syn gas, carbon dioxide, hydrogen, carbon monoxide (as well as methane, ethane and propane were generated, all in appreciable quantities.
Of course, there is no intrinsic reason to limit temperatures to 500°C in an age where thermal barrier materials exist and where refractory and machinable metalloceramics, many carbide based, are known.
At 1400°C, the temperature at which the cerium oxide based system that I discussed in this space and linked above decomposes both or either steam and/or carbon dioxide into carbon monoxide or hydrogen, the lower saline phase in supercritical seawater would be above the melting point of all the salts therein and, in the case of super critical seawater from the Mississippi Delta, all of the algae, all of the microplastics, all of the larger pieces of plastic, all of the residue of the Deepwater Horizon disaster that took place while we all waited lazily for the so called “renewable energy” nirvana that never came, is not coming and will not come, would be rendered into hydrogen and carbon oxides – syn gas – all most of of the phosphorous from the grand renewable corn ethanol scheme that destroyed the delta would be recovered. The superheated flows of steam, carbon oxides and hydrogen would be available for all kinds of process intensification schemes, distillation of fuels for those mobile devices that would be wise to keep (as opposed to cars), rescue helicopters, freight vehicles, tractors, ambulances, etc, including the wonder fuel DME and potential lubricity agents like the OME3-5 discussed above.
There are, I note, hundreds of possible known thermochemical cycles for splitting carbon dioxide or water, some involving metals like zinc, copper, cerium and the alkali metals, as well as inorganic species as in the sulfur iodine cycle and variants therein. For amusement, I dream up some of my own.
Seawater, by the way, contains about 60 times more carbon dioxide on a weight basis than air, and thus the ocean would be an extractor for commercially recoverable carbon dioxide and it would be far more thermodynamically feasible, and thus environmentally and economically feasible to remove carbon dioxide from the air via seawater.
As for the nuclear fuels themselves, one of the things we should have learned at Chernobyl and Fukushima is that the most problematic fission products are those which are volatile and that it is possible to distill, and under appropriate conditions collect them to do useful things. In fact by careful timing of the transfer to various containers during decay of short lived radioactive xenon isotopes, one can obtain isotopically separated cesium isotopes, and collect valuable and expensive stable xenon isotopes for lighting and other applications.
By extension, every energy related mine in the world could be shut, coal mines, fracking fields for oil and gas, offshore oil and gas wells. It is possible, in fact, to shut all of the world's uranium mines if we build a new type of brilliant reactor concept advanced most prominently by the Japanese nuclear engineer Hiroshi Sekimoto, the "CANDLE" concept, "CANDLE" being a somewhat tortured acronym from "Constant Axial shape of Neutron flux, nuclide densities and power shape During Life of Energy producing reactor." Application of candle burnup strategy for future nuclear energy utilization (Hiroshi Sekimoto, Progress in Nuclear Energy; Vol. 47, No. 1-4, pp. 91-98, 2005). Many variants of this concept have been advanced, the most notable being Bill Gates' "Traveling Wave Reactor" put forth by his company Terrapower, which is the closest to commercialization.
Under these conditions the uranium and thorium already mined - the latter being contained in the mine tailings for the lanthanides we use for our wonderful electric cars and our wonderful wind turbines - would be sufficient to address all the world's energy needs for centuries, since depleted uranium and dumped thorium will be translated from "waste" into valuable fuels.
When I consider my own versions of "breed and burn" biphasic reactors - not that anyone cares what I think - I evaluate them in terms of on line processing, involving nitride fuels, uranium nitride for those sparked by isotopically degraded plutonium, and thorium nitride for those sparked by either neptunium and americium, I consider their potential for on line reprocessing, for the on line recovery of valuable fission products which, if allowed to escape, as at Chernobyl or Fukushima, are problematic, although the health consequences of these two accidents are dwarfed by the health consequences of dangerous fossil fuel waste.
Everything I've written here flies in the face of what has popularly come to represent modern liberalism, but I am a throwback to another time, a time when protecting natural spaces from development was more important than converting them into industrial parks for wind turbines to power electric cars for bourgeois consumers, a time that liberalism involved caring about the fate and lives of the poorest of the poor rather than demanding that these people be enslaved to dig cobalt for lithium batteries, a time when human advancement in a truly sustainable with equal justice and equal opportunity.
This is not the world in which I live anymore.
As for energy and the environment, I flatter myself to reflect on the early environmentalist John Muir, founder of the Sierra Club (which has evolved in modern times into a promoter of wind industrial parks in the middle of forests and marine ecosystems), who fought against the industrialization of the Hetch Hetchy valley and lost; the valley has been lost forever with the building of a hydroelectric dam.
Glenn Seaborg, scientist, diplomat and administrator, was famously a Democrat, and we, the modern Democrats, have forgotten that entirely how to respect his work.
Nevertheless, all this aside, as I approach the end of my life, these things matter to me, and I feel compelled to write them down, and to learn by writing about them, as I have here, even if they are too long, too turgid, and too technical for anyone to actually read.
In Greek mythology, Cassandra, the daughter of King Priam of Troy was cursed to be able to know the future, to understand it perfectly including the destruction of her city state, and to predict it all, but to not be believed. It was a terrible curse but she did what she could, and I love the thought of her as I love myself. Her fate was to be raped, while clinging to the image of her god, and eventually murdered. That won't happen to me, but still...

Ajax and Cassandra, 1806, Johann Heinrich Wilhelm Tischbein (1751–1829) at the Großherzogliches Schloss Eutin, Germany.
I trust readers, if any, will enjoy a pleasant Sunday, and perhaps think happy thoughts when viewing the cartoon at the beginning of this post.
Loki Liesmith
(4,602 posts)But I think you’d be better served breaking these up into several posts.
NNadir
(37,816 posts)Last edited Sun Apr 28, 2019, 11:40 AM - Edit history (1)
In my times, people were willing to read long texts to understand things.
I am, I confess, of another age.
Loki Liesmith
(4,602 posts)For human interpretability. For whatever reason I’ve found that discrete physical pages are better for information retention than the continuously scrollable computer (or phone) screen.
Regardless this is very good work and thanks for putting the pieces together.
NNadir
(37,816 posts)...there's the convenience of "live links" on the computer.
Each have their pluses in minuses. It's easy to carry a book or a paper journal around the house; to read oneself to sleep in bed.
One can feel a little more intimate with a work on paper.
There is no way, however, that I could possibly fit the library I have on disk in the form of paper, most of which I have downloaded at University libraries at the relatively low cost of access.
In the days of paper journals, even the few to which I subscribed, ended up filling a room, and it was impossible to search them as quickly and as facilely as I can search now. I recall the pain of dumping years and years of journals, pulling the more recent years off the shelves and dumping them into the recycling bin and carrying them down from boxes in the attic, something my wife insisted had to be done, but I haven't missed them. It's easier and more powerful to spend a couple of hours in the library and carry everything home on a tiny disk attached to a lanyard I keep around my neck.
I lost, of course, the sort of random learning one gets when one is leafing through paper pages and some random topic catches one's eyes, and worried about losing that when things went electronic; but I find that just scrolling through a table of contents gives something very close to that.
In any case, thanks for reading this. I really don't expect many readers, but post them because I learn so much for myself I writing them, and the off chance that someone else finds them worthwhile, that's a bonus.
The intense study of the issues of climate change is depressing because I feel that almost no one actually gets it; our denial on the left about how to address climate change is having the same effect as the denial on the right as to whether climate change is actually real, even if we can see and endure it with our own eyes.
I've convinced myself that those of us on the left are as dangerous as they are on the right when it comes to the denial of the facts.
It's going to get much worse before it gets better, and even though I won't live to see the worst of it; I do care about the future generations as my parents cared for mine.
Thanks again.
hunter
(40,609 posts)Alas, it's not a Happy Meal, or even something you could buy at a favorite organic food store and bring home in a compostable container.
I was intrigued by your mention of desalinization. Making fresh water from seawater is another process that becomes much more difficult when the primary energy source is intermittent, as it always will be with wind and solar.
NNadir
(37,816 posts)The talk is on line. It can be accessed here: Science on Saturday: Managing Coastal Risk in an Age of Sea-level Rise. Scroll down for the lecture video.
One of the things Bob Kopp covered in the lecture is something I've been wondering about for some time, which is to what extent does the mining of fossil water, as in the Ogallala Aquifer, on which a significant portion of US grain production depends effect sea levels.
According to him - and he's a fascinating thinker - about 10% of the currently observed rise in sea levels is attributed to the mining of fossil water and it's release into the hydrosphere.
One one hand, I'm concerned about the effects of saline gradients on ecosystems, on the other hand, the seas are being diluted by climate change, the change of saline gradients is out of control with or without desalination.
Some compromises are going to need to be made.
There are good reasons why I think that supercritical water desalination in the Gulf of Mexico, for one place, is a very good idea, for the reasons I gave in the OP. The water there is in trouble and it the quality of the water can be greatly improved by supercritical water oxidation. Of course, they don't necessarily need water in Louisiana, at least most of the time, and as we know, they often have way too much of it. On the other hand it might be pumped or sucked to places where it is needed.
I personally think that process intensification, as is possible with supercritical water desalination, can produce very high efficiency energy use which can, among other things, drive pumps on what was once considered waste energy.
However, from where I stand, the only possible approach to cleaning the air of the dangerous fossil fuel waste carbon dioxide must go through extraction and concentration in seawater.
It is also an approach to making uranium supplies essentially infinite, and in a moral world, each generation would extract exactly what it uses. However uranium requirements to power the whole world are rather small. Suppose we decided to raise world energy consumption to 1000 exajoules a year in an effort to clean up the mess of carbon dioxide waste in our atmosphere, eliminate human poverty and do a host of other useful and good things. It can be shown that this would require the fission of 395 grams of plutonium each second, or about 12,500 tons per year, which is less than the amount of unused uranium already in US used fabricated nuclear fuel.
At the accepted concentration of uranium in seawater, this would involve processing 3 trillion cubic meters, slightly less than the volume of Lake Michigan, but the main things to be recovered would be water and carbon.
This sounds like a lot of water, until one realizes that the State of Kansas, for example irrigates with about 4 billion cubic meters every year, much of it coming from the increasingly depleted Ogallala. With this in mind, it's very clear that world fresh water demand is easily on a trillion cubic meter per year scale.
There are many places all over the world where water supplies are severely threatened, particularly, over the long term the vastly populated portions of Asia that have depended on Himalayan glaciers, glaciers that are threatened. Of course, it may be absurd to discuss this today with much of Nebraska recovering from massive flooding, but to the extent we have a mechanism for moving vast sums of water, we might manage our survival better.
To the extent that depleted fossil water reserves are replenished, like, but certainly not limited to the Ogallala, this will serve to slow sea level rise, apparently, if Dr. Kopp is to believed, by an appreciable, if minor, amount. However coupled with the removal of carbon dioxide from the air, it might do something. The big things though, are glaciers and ice caps.
Right now, except for the cheer leading for solar and wind and hyping electric cars, nothing useful is happening.
The key to recovering from the energy waste situation is, in fact, energy, but energy with a low pollution profile, the only one to have worked, to be scalable, and to be sustainable, being nuclear energy.
This is all a little dreamy, I know, but it's the most practically approachable thing that seems even remotely feasible. It's a very challenging task, and no, getting one's underwear in a wedgie thinking about Elon Musk and wind turbines is not going to cut it. I have no illusions that these things will be done, but I believe that they are possible. I am reluctant to use the word "could" as is often used here with the word "study" to claim for example, "Greenpeace study shows that the world could be powered 100% renewable energy [insert year du jour here." Still, while the possible is often improbable, everything that is was once improbable.
The issue is scale. I don't think we have any idea of the scale of the disaster we are leaving for all future generations.
We will not be forgiven, nor should we be.
John ONeill
(87 posts)Your mention of liking nitride fuels lead me to look at how the Russians were doing on that, as the only ones with any actual fast neutron reactors in service. As I'm sure you know, the BN600 and BN800 run on oxide fuels, UO2 or MOX, but the plan is to eventually move to nitride, or possibly metal fuels. From what I could find, the burnup of nitride fuels they've tried is limited by fuel swelling, or interaction with the rod cladding
-'The performance of nitride fuel pin as well as oxide is limited by irradiation stability of cladding steel, and also by fuel-cladding mechanical interaction (BR-10 reactor experience).The positive BORA-BORA results confirm the possibility to provide at least12at% of burn-up for He-bonded pins at initial fuel porosity increase. '
https://www-pub.iaea.org/mtcd/meetings/PDFplus/2009/cn176/cn176_Presentations/plenary_session_5/INV-02.Zabudko.pdf
I'm surprised that they're using helium for the thermal bond between the fuel meat and the cladding. The USA's Experimental Breeder Reactor II used sodium bonding very succesfully with metal fuels, since the superior thermal conductivity of sodium allows a wider clearance between the fuel and the clad, giving more room for swelling without damage to the rods. Sodium bonding was also considered for light water reactors, as it should keep the rods cooler, but presumably vetoed in case it leaked into the water coolant. For sodium cooled reactors that wouldn't be an issue, so is there some other reason not to use it with UN or PuN ?
Great post, by the way. Go as long as you need to. John O'Neill
NNadir
(37,816 posts)...that to my knowledge has not been explored in any way. Nitrides have certain chemical properties that make them interesting for online recovery of valuable fission products. The thinking is rather elaborate, but you'll have to take my word for it that I believe the cladding an swelling issues that dog many high burn up reactors could use some rethinking.
NNadir
(37,816 posts)It is probably of no interest, but since I spent a lot of time thinking about these papers and writing about them, I thought I'd note it.
The corrections are here: Corrigendum: “Production of Oxymethylene Dimethyl Ethers from Hydrogen and Carbon Dioxide–Part I: Modeling and Analysis for OME1 & Part II: Modeling and Analysis for OME3–5” (Jannik Burre, Dominik Bongartz, and Alexander Mitsos Industrial & Engineering Chemistry Research 2020 59 (49), 21615-2161)