Environment & Energy
Related: About this forumThe Role of Copper Oxides in the Electrochemical Reduction of CO2 to C2 Carbon Fuels.
As I often point out, electricity is a thermodynamically degraded form of energy, and storing it as chemical energy degrades it even further. The caveat to this statement is that where electricity generation is a side product of another process involving high temperatures, such as the thermochemical production of hydrogen, or, as I recently discussed in this space, zero discharge supercritical water desalination. Under these circumstances, electricity, which may represent waste electricity if demand is low, can be utilized to increase the exergy of a system even if there is a thermodynamic loss associated with the conversion of electricity to chemical energy.
Here is the zero discharge desalination scheme I discussed fairly recently, in some detail, a situation in which electricity might be a side product of another process: The Energy Required to Supply California's Water with Zero Discharge Supercritical Desalination.
A great deal has been written about the electrochemical reduction of carbon dioxide to give various hydrocarbons, alcohols, aldehydes and organic acids - the latter most often formic acid. Recently I came across a nice review of the topic: Modeling Operando Electrochemical CO2 Reduction Federico Dattila, Ranga Rohit Seemakurthi, Yecheng Zhou, and Núria López Chemical Reviews 2022 122 (12), 11085-11130
Here is an intriguing graphic from the paper:

The caption:
Reference 129 is this one: Guiji Liu , Michelle Lee, Soonho Kwon, Guosong Zeng, Johanna Eichhorn, Aya K. Buckley, F. Dean Toste , William A. Goddard III , and Francesca M. Toma CO2 reduction on pure Cu produces only H2 after subsurface O is depleted: Theory and experiment, PNAS 118 (23) (2021) e2012649118
Here's a graphic from this paper:
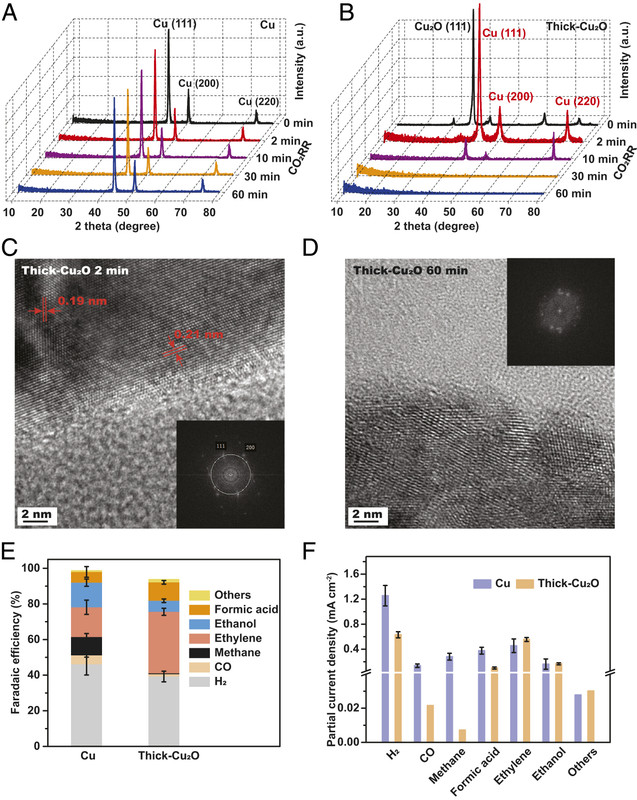
The caption:
It appears that copper (I) oxide is essential for producing hydrocarbons electrochemically and improves the yield over the production of hydrogen. Once the oxide is reduced, the product is more or less just hydrogen. The electrodes can be regenerated by simply exposing them to air, albeit for extended periods.
Regrettably I don't have much time to go into the details.
Hydrogen of course, can be converted to useful fuels via Fischer Tropsch chemistry (for petroleum like fuels) or fuels superior to petroleum, the best of which is dimethyl ether, a highly flexible and easily transportable fuel. It is not, however, despite decades of stupid rhetoric that still goes on and on and on and on, a useful consumer fuel: The infrastructure for so utilizing it would be expensive, unsustainable, and frankly dangerous. Hydrogen use should be limited to its already important realm as a useful captive intermediate in the chemical industry.
Direct reduction of carbonates to carbon fuels with water as the hydrogen donor is probably a good idea, again, only under the thermodynamic constraint that the electricity so utilized is a side product of other processes, represented "captured" exergy, available to be dispatched to grids on an "as needed basis."
Note that Faradaic efficiency is not the same as thermodynamic efficiency. Faradaic efficiency can be thought of as the fraction of electrons that end up in the product or in this case the products. The overall thermodynamic efficiency can be thought of as the product of the Faradaic efficiency and the Voltage efficiency, the latter representing the overvoltage required to drive the reaction. Essentially this is the extra energy to over come electrical resistance in the systems and represents energy lost as heat.
It will be interesting to see how nanostructured materials may be applied to further move these kinds of systems to ethylene to sequester carbon as polymeric material and to eliminate the use of dangerous fossil fuels.
Have a nice day tomorrow.
hunter
(40,481 posts)... are turned into plastic pipe.
Every human, all eight billion of us, deserves clean water and indoor plumbing. This can be accomplished using polyethylene pipe.
Wealthy people currently use a lot of copper inappropriately for things like electric cars, wind turbines, indoor plumbing, etc.. The negative environmental impacts of mining and refining this copper are great.
Ultimately the most important use for copper will be as a catalyst.
eppur_se_muova
(41,327 posts)Quite a career change.
NNadir
(37,550 posts)...show his authorship of a number of papers that clearly involve experiment, but generally the authorship is broad. Perhaps his role is strictly to provide a theoretical basis for experimental results.
It may not have been a career change so much as a willingness to collaborate with experimentalists, which is a good thing.
But again, I'm largely unfamiliar with his work.
It is rare that a great scientist's career shows expertise on both sides. The most famous exception of course, was Enrico Fermi.
I believe an outstanding theoretician must engage experiments, and all of the greatest theories began with an experimental observation.
By contrast, an experimentalist who is surprised by his or her own results will need to call on a theoretician in most cases to justify that the experimental results. Sometimes of course, theory is thrown away because of experiment. The case that comes to mind is the Michaelson-Morley experiment to "measure" the speed of the "ether." It took some time, and in fact, Albert Einstein to come up with a way of explaining that one. Einstein, I've heard, was a horrible experimentalist.
eppur_se_muova
(41,327 posts)Every picture of him found in a quick Google search shows him still wearing the beret, so that appears to be a constant.
Apparently his formal title changed -- he is no longer a Prof. of Theo. Chem., but of Chem. and Applied Phys. So that seems a little out of the usual.
I suppose you've heard of the Pauli Effect. I was most surprised to read that some people, including Pauli, took it seriously.
***
Background
Since the 20th century, the work in some subfields of physics research has been divided between theorists and experimentalists. Those theorists who lack an aptitude or interest in experimental work have on occasion earned a reputation for accidentally breaking experimental equipment. Pauli was exceptional in this regard: it was postulated that he was such a good theorist that any experiments would be compromised by virtue of his presence in the vicinity. For fear of the Pauli effect, experimental physicist Otto Stern banned Pauli from his laboratory located in Hamburg despite their friendship. ...
***
An incident occurred in the physics laboratory at the University of Göttingen. An expensive measuring device, for no apparent reason, suddenly stopped working, although Pauli was in fact absent. James Franck, the director of the institute, reported the incident to his colleague Pauli in Zürich with the humorous remark that at least this time Pauli was innocent. However, it turned out that Pauli had been on a railway journey to Zürich and had switched trains in the Göttingen rail station at about the time of the failure. The incident is reported in George Gamow's book Thirty Years That Shook Physics,[7] where it is also claimed the more talented the theoretical physicist, the stronger the effect.
R. Peierls describes a case when at one reception this effect was to be parodied by deliberately crashing a chandelier upon Pauli's entrance. The chandelier was suspended on a rope to be released, but it stuck instead, thus becoming a real example of the Pauli effect.
***
https://en.wikipedia.org/wiki/Pauli_effect
