Science
Related: About this forumA Review Article On the Utilization of Carbon Dioxide.
As I often repeat, the failure of humanity to address the destruction of the planetary atmosphere will require future generations, should they prove capable of restoring whatever is left to restore of this planet, will require the removal of carbon dioxide from air, possibly via its removal from seawater.
This is a huge thermodynamic, and therefore engineering and energy challenge. It will require future generations to produce more energy than we now consume profligately, and with zero interest in the waste this energy production involves.
Of course in dealing with cleaning up our waste, future generations will be belabored and not enriched, but it's clear we couldn't care less about them, so it's their problem.
In this sense we are all Republicans, whether we acknowledge it or not; we care only about ourselves and have no interest in the welfare of other people. In this case, when I refer to "other people," I am referring to people who are now infants or children, and their children and infants, that is, all future generations.
Of course, it is not enough simply to remove carbon dioxide from the air; one must also have a place to put it.
The "place to put it" has often been imagined with the endless proposals of waste dumps euphemistically called "sequestration," although the word "dump" is appropriate. The current waste dump is of course, the atmosphere.
It turns out that another means of dealing with carbon dioxide would be to utilize it, which is a proposal to make it something other than "waste." Carbon dioxide is a currently utilized product industrially, although its use currently is nothing like the quantities dumped into the atmospheric waste dump, an amount on the order of 35 billion tons per year.
I think about these things a lot, and this is why I was pleased to go through the recent issue of Chemical Reviews, which was about sustainable chemistry.
An aside:
Recently in this space, in a post to which this post is a follow up, a comment of Vaclav Smil's (I wish people would embrace his clear thinking about energy, if not necessarily accepting his conclusions) about how refining of steel requires coal - I would argue that it need not do so forever, as I will briefly allude to below - that I partially repeat:
To answer the part I have put in bold above, my own definition of sustainability, if clearly not that of my contemporaries, would be a statement that each generation leaves for subsequent generations a planet that will afford ecosystem of the planet more or less in the same state into which they were born, and will allow the members of future generations the same level life style - including the opportunity to appreciate the beauty of the natural world - that the generation leaving enjoyed.
And no, the construction of millions of wind turbines that will be rotting hulks 30 years after construction, and millions of solar cells that will be electronic waste in thirty years won't cut it, any more than spent fracking fields - on which the fantasy to the contrary has depended, is depending and will always depend - leaching radioactive and chemically contaminated flow back water for centuries will cut it.
The paper I will discuss is this one: Sustainable Conversion of Carbon Dioxide: An Integrated Review of Catalysis and Life Cycle Assessment (Leitner et al, Chem. Rev., 2018, 118 (2), pp 434–504)
The authors are German, and well they should consider this point, since the official and in my opinion extremely ignorant national German energy policy is to put lipstick on the pig of long term fossil fuel dependence which they are clearly interested in entrenching forever, or at least until every trace of fossil fuel waste that can be generated has been stuffed into the atmosphere and oceans, neither of which clearly can take it anymore without severe changes to their stability.
The paper's opening graphic, which I believe is accessible from the abstract is this one:

It is slightly inaccurate, since it seems to show a requirement for free isolated hydrogen, which is actually not necessary, although hydrogen might be present in many - maybe in most - schemes as a captive intermediate.
There are ways that carbon might be converted into products that do not depend on hydrogen, as I will describe briefly below.
"Life Cycle Analysis," "LCA," is an increasingly important discipline in evaluating the "sustainability" of a particular energy practice, but it is information dependent inasmuch as it depends on intimate knowledge of industrial processes. It is important to consider that it is also necessarily subjective.
For example it might matter to me if formerly pristine deserts are strewn with rotting metal thirty years from now as represented by abandoned wind farms, but it is not clear that if you establish the criteria as being "loss to human life" as being the only criteria that matters, the rotting metal abandoned wind farms will not be as important - although the production of the steel in them will have lead to losses of human life in the generation that built them because modern steel production always utilizes coke which is almost always made by heating coal.
Here is another graphic from the internal text of the paper that puts things in life cycle terms:

Here is the caption for that graphic:
Reference 117, Life cycle assessment of polyols for polyurethane production using CO2 as feedstock: insights from an industrial case study (Barlow and Assen, Green Chem., 2014, 16, 3272 does not contain the graphic immediately above, although the abstract shows a graphic present in the paper itself shows propylene oxide reacting with carbon dioxide to give a polymer. (Not shown is the solvent, which is DMC, dimethyl carbonate, which can also be made from carbon dioxide, as the review article discusses at length.)
Reference 117 is about a polycarbonate, a type of plastic which in this case is utilized to produce another polymer, polyurethane, albeit using a product obtained from dangerous fossil fuels, toluene isocyanate. (cf Green Chem, 2016, 16, 1865)
Returning however to the graphic above, note that it is an octagonal representation with lines from each of the vertices to the center representing a "reference case" for an environmental impact, and that the "global warming" impact is merely reduced, by less than 20%, not eliminated.
In reference 117 the process is described as capturing carbon dioxide from lignite coal burned in Germany's lignite coal plant at Niederaussem which is not being phased out like Germany's nuclear plants - all to be phased out - that do not require fossil fuels and which do not dump fossil fuel waste into the atmosphere.
Moreover, it's not clear that the carbon dioxide to make the polymer will never be added to the atmosphere, and quite possibly the whole enterprise, as described, is lipstick on the coal pig.
This said, were the carbon dioxide obtained from the removal of carbon dioxide from the atmosphere by carbon dioxide free means - which in my view can only be represented by nuclear energy, the following graphic from the review, a representation of the "savings" (with respect to direct dangerous fossil fuel waste dumping) would have a different form:

To wit, the denominator in the unit for the ordinate would disappear, since no carbon dioxide would be produced to make the polymer. To the extent that the polymer were recycled (albeit requiring an investment of energy) it would be possible to entirely close the carbon dioxide cycle, or at least minimize by a factor of perhaps 90% as opposed to "less than 20%."
(Don't you love "percent talk?" I actually don't, since it's most often used, in particular in the misrepresentation of the solar and wind industries as significant - which they are not when compared to the rapidly increasing use of dangerous fossil fuels, to perpetuate a lie rather than to describe the truth.)
Now let's turn to some chemistry, with this graphic from the review:

Reaction set (a) is lipstick on the methane (dangerous natural gas) pig, and refers to the mistaken impression that hydrogen is a "green" or "clean" fuel. This reaction - and it's coal based analogue - is, by the way, the means with almost 99% of the hydrogen industrially produced on the planet, by reformation, and not by electrolysis, the stupid exercise on the Norwegian Island of Utsira notwithstanding.
Reaction (b) is the partial reversal of reaction (a) and is known as the "water gas shift" reaction.
Reaction (c) is part of the international pipe dream about the wind and solar industries; it is extremely thermodynamically inefficient and the dream of expanding it to meaningful levels using technologies that have never proved meaningful, solar and wind, is in fact simply a measure of denial as pernicious as the level of denial practiced by the idiots in the Republican party.
Reaction (d) is of some interest depending on whence the high temperatures required come; provided by nuclear means, they can significantly improve the thermodynamic nightmare of electrolysis; produced by the solar thermal day dream - that industrially is environmentally destructive and totally dependent on supplemental dangerous gas as well as exorbitant in cost - it's just more garbage thinking.
Reaction set (e) is however quite interesting if only represented by loose schematic, non-stoichiometric carbon dioxide and water splitting.
In the modern scientific literature, this set (e) is almost always described in terms of "solar thermal" schemes. These do not work and will not work for the simple reason that batch processes are always more expensive and always dirtier than continuous processes, a fact that should be familiar to any well educated chemical engineer.
Historically these processes were often described by nuclear systems, and the most famous thermochemical hydrogen cycle, the sulfur iodine cycle, was that which was pushed by the General Atomics company, a company that proposed to build HTGC reactors with a helium working fluid Brayton cycle which would, in theory cogenerate hydrogen by splitting water.
The sulfur iodine cycle is not represented by the series in equations (e), nor should it be, since reactions (e) can produce either hydrogen or carbon monoxide, the latter being utilized by the use of reactions in set (a) to produce hydrogen without dangerous fossil fuels.
The GA HTGC reactors proved to be economic failures, since the attempt to build them - a few were built but didn't operate all that long - was before its time: The successful production of sustainable refractories advanced in the 1960's and 1970s in connection with the Space Program as a side product of that noble enterprise.
It is worth noting by the way, the General Atomics is now the site of one of two research nuclear fuels reactors in the United States, the other being at the Princeton Plasma Physics lab outside of Princeton, NJ.
If fusion ever became a practical form of energy - I doubt it will be significant in the lifetime of anyone now living, any more than solar or wind energy will be - all the reactions in set (e) would be practically and moreover sustainably driven.
About CO, carbon monoxide: Methane is often incorrectly thought to be "clean burning" because it produces low particulates, lacking the carbon-carbon bonds found in the admittedly dirtier fuels petroleum and coal.
The oxidation of methane does not, however, only produce the dangerous fossil fuel waste carbon dioxde since it is almost always the case that the oxidation is incomplete, and one incomplete product of said oxidation is carbon monoxide. Indeed the industrial process for producing hydrogen does this deliberately, partially oxidize methane.
Carbon monoxide however is not thermodynamically stable at all temperatures, it in fact exists in equilibrium with pure elemental carbon and carbon dioxide. This is known as the Boudouard equilibrium:
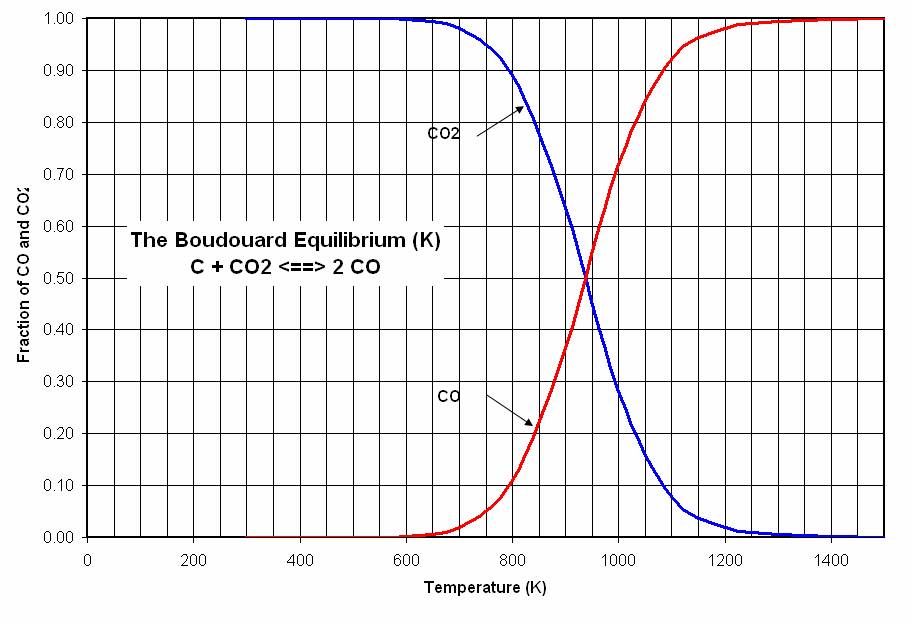
The Boudouard equilibrium shows that it is possible to obtain carbon from carbon dioxide, should one split carbon dioxide by reactions like those in reaction series (e), isolate carbon monoxide resulting from it, and then drive the Boudouard equilibrium to carbon by removing carbon dioxide by simple thermally reversible chemical means.
One of the tools for producing steel is carbon, whether the steel is structural steel in skyscrapers, or cars, or bridges or for that matter the quixotic enterprise of building windmills that pass, inappropriately, for energy decency in these times.
Theoretically at least, it may not thus be true that in order to make steel, one needs to mine coal.
And to the extent that metal carbides are used as important materials, and to the extent that other carbon based materials like those highly involved in modern nanotechnologies for just one example they represent utilized carbon that is not in the atmosphere.
A great deal of the review article covers the preparation of motor fuels from carbon dioxide. These are in general dependent on generating hydrogen, although some of the work covers electrolytic means of reduction, for example the electrolytic reduction of carbon dioxide to methanol, formaldehyde, formic acid and even alkanes.
Hydrogenation produces - I suspect with far greater thermodynamic efficiency - fuels like DMC, dimethyl carbonate, and the wonder fuel dimethyl ether, which for my money is the best energy storage material possible, far superior to hydrogen itself.
By reactions like those in set (e) above, all of these things can be accomplished by nuclear energy.
Is this easy? No, it isn't. There's no reason to be as glib as the failed "solar will save us" and "wind will save us" nonsense rhetoirc one hears all the time.
However if we are having a sustainable world, we are a long way away, and as everything we've done has failed, it's to try another way.
Below are some additional graphics from the review, which, being a review article discussing other papers, may or may not be broadly applicable. It is worth noting that many publications do not discuss nuclear energy, because nuclear energy is subject to broad public, if ignorant, approbation, and public approbation and attitudes do in fact, rightly or wrongly, effect the issuance of the grant system that supports our science.
A big graphic of the water gas equilibrium:

This graphic refers to "DRM" or dry reforming of methane, in which the dangerous fossil fuel natural gas is oxidized using CO2 rather than oxygen, a half-assed approach to eliminating carbon dioxide waste, but still an improvement in the efficiency of its use.:

The caption:
From my perspective, it is not enough to reduce carbon dioxide dumping, but it is essential to not dump it at all.
Here's a graphic representing the catalysts for "DRM:"

Here's a big blow up of the equations for metal based thermochemical carbon dioxide and water splitting. Metals included in these schemes are typically, if one wanders around the literature, iron, cerium, and (albeit not represented by the equations here, tin. There are other examples.

This graphic is a schematic of high temperature and low temperature electrolysis of water and carbon dioxide, performed in some cases synergistically.

This graphic refers to the production of the hydrogen storage compound formic acid, and regrettably refers to the use of dangerous fossil fuels, and is thus not a fossil fuel elimination scheme so much as a reduction scheme, and therefore in my personal view, not sustainable.

Some text from the review - there's lots of text - that describes an interesting and beautiful formic acid production scheme, albeit one that still needs work:
Whatever the limitations of the schemes found in this review, if you can find access, the review is worth a read.
Have a pleasant Sunday evening.
