An interesting discourse on the biological marine sulfur cycle.
The paper I'll discuss in this post is this one: The metabolite dimethylsulfoxonium propionate extends the marine organosulfur cycle (Kathleen Thume, Björn Gebser, Liang Chen, Nils Meyer, David J. Kieber & Georg Pohnert, (Nature Volume 563, pages 412–415 (2018))
When I was a kid, one of my first professional successes in the lab involved the hydrophobic amino acid methionine, which is one of two of the 20 proteogenic amino acids (21 if one counts bacteria) for which genetic codons exist. Methionine is one of two members of this class which contains sulfur, the other being cysteine.
The role of cysteine, a thiol, in proteomics is spectacular, since its oxidative interaction with other cysteines in proteins is responsible for disulfide bridges without which many proteins would be useless, lacking the requisite geometry, and almost equally important, its role in metal co-ordination at catalytic centers of very important proteins. Methionine, a thioether rather than a thiol is far more rare in proteins, although it is important in the transfer of methyl groups in biological interactions and it has recently been discovered that interactions with the π systems in phenylalanine, tyrosine and - who knows - histidine also serve to stabilize protein geometry. Bacterial methionine biosynthesis (Ferla and Patrick, Microbiology (2014), 160, 1571–1584).
I am also interested in the chemistry of sulfur, because my generation failed all future generations by turning out planetary atmosphere into a vast waste dump for the dangerous fossil fuel waste carbon dioxide, and I am always thinking about ways that future generations can clean up our mess, and do so with a functionally destroyed resource base consisting almost entirely of our solid phase garbage dumps. One path to removing carbon dioxide from the atmosphere is to essentially reverse combustion by making the source of carbon materials - now made from dangerous fossil fuels - carbon oxides obtained by the thermal reformation (gasification) of waste (or problematic) biomass, using nuclear energy as the primary energy source. These technologies are being widely explored but a consistent factor that engineers must address is the fate of heteroatoms in biomass like potassium, sodium and, relevant to this discussion, sulfur.
Finally the paper cited at the outset of this post caught my eye because I always wonder about the environmental fate of common laboratory chemicals - one of which is dimethylsulfoxide - a wonderful solvent with wide use - and also because of my interest in stable charged organic species that play a role in a rapidly developing area of chemistry, ionic liquids.
So the paper itself:
From the abstract:
Algae produce massive amounts of dimethylsulfoniopropionate (DMSP), which fuel the organosulfur cycle1,2. On a global scale, several petagrams of this sulfur species are produced annually, thereby driving fundamental processes and the marine food web1. An important DMSP transformation product is dimethylsulfide, which can be either emitted to the atmosphere3,4 or oxidized to dimethylsulfoxide (DMSO) and other products5...
So it turns out that DMSO, which is used in some products for joint pain, is a normal biological species, present in the oceans on what may be a million ton scale. (I had a neighbor - to whom I stopped speaking years ago - who called me up to ask me if he could sue his employer because they asked him to work with this chemical, but that's another story.) That makes me feel better about all the DMSO I've used - or asked scientists working for or with me to use - in my career, for solvation, Swern oxidations, blah, blah, blah...
From the introduction to the paper:
The marine organosulfur cycle is fuelled by small sulfur-containing zwitterionic osmolytes that are primarily produced by planktonic algae. The main metabolite of this class, DMSP, is produced in the impressive amounts of 2 petagrams (2 × 109 tons) sulfur annually1. Cellular DMSP serves important physiological functions in marine algae that include, but are not limited to, acting as an osmolyte, a cryoprotectant and an antioxidant6,7. Enzymatic lysis of DMSP by DMSP lyases in bacteria and algae yields acrylate and dimethylsulfide (DMS)8. Volatile DMS is the main source of organosulfur in the atmosphere; and with an annual flux of approximately 30 teragrams of sulfur3, DMS has been proposed to affect cloud formation and regulate climate4. Dissolved DMSP arising from exudation, grazing, viral lysis and cell mortality serves as substrate for marine microorganisms7,9,10. In surface waters, substantial quantities of dissolved DMSP and DMS can be detected, but often the concentration of dissolved DMSO exceeds the concentration of each of these two species5,11. DMSO is mainly produced from bacterial and photochemical DMS oxidation12, but algal sources of DMSO may also be important13 The marine organosulfur cycle is fuelled by small sulfur-containing zwitterionic osmolytes that are primarily produced by planktonic algae. The main metabolite of this class, DMSP, is produced in the impressive amounts of 2 petagrams (2 × 109 tons) sulfur annually1. Cellular DMSP serves important physiological functions in marine algae that include, but are not limited to, acting as an osmolyte, a cryoprotectant and an antioxidant6,7. Enzymatic lysis of DMSP by DMSP lyases in bacteria and algae yields acrylate and dimethylsulfide (DMS)8. Volatile DMS is the main source of organosulfur in the atmosphere; and with an annual flux of approximately 30 teragrams of sulfur3, DMS has been proposed to affect cloud formation and regulate climate4. Dissolved DMSP arising from exudation, grazing, viral lysis and cell mortality serves as substrate for marine microorganisms7,9,10. In surface waters, substantial quantities of dissolved DMSP and DMS can be detected, but often the concentration of dissolved DMSO exceeds the concentration of each of these two species5,11. DMSO is mainly produced from bacterial and photochemical DMS oxidation12, but algal sources of DMSO may also be important13. Common pelagic bacteria use monooxygenases to oxidize DMS to DMSO14, a process that may serve as an energy source15. Here we report on the identification of the zwitterionic metabolite DMSOP, which is widely distributed in phytoplankton and also produced by marine bacteria. This metabolite is the substrate of a previously undescribed marine pathway for DMSO production (Fig. 1) .
It's probably now time to just look at the pictures the authors use to describe their "zwitterionic" species. (A zwitterion is a molecule that possesses, in the same molecule, both a positively and a negatively charged ion. The important physiological molecule carnitine is an example of a zwitterion.)
A picture:
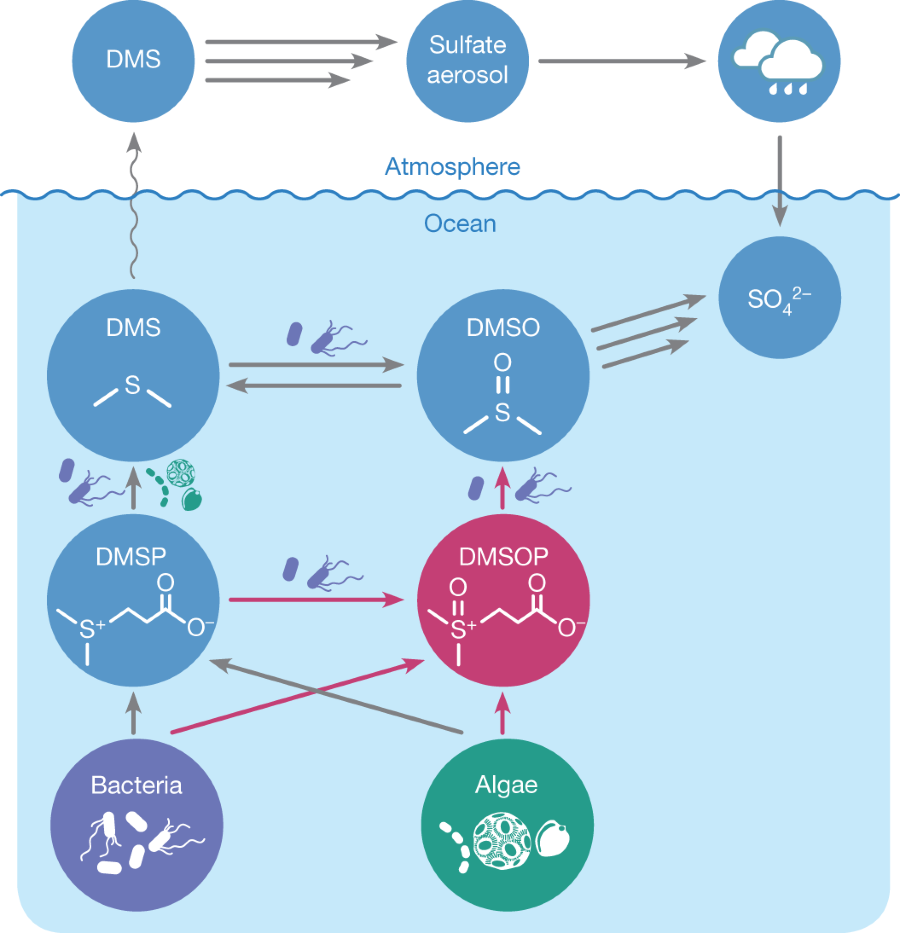
The caption:
DMSOP and the transformations labelled with red arrows extend the established marine sulfur cycle. DMSOP is produced in eukaryotic microalgae (green) as well as in bacteria (purple). Bacteria metabolize DMSOP and therefore contribute to the marine DMSO pool. The established DMSP-based part of the sulfur cycle is indicated with grey arrows. DMSP is formed by marine algae and bacteria. It is then cleaved by algal and bacterial DMSP lyases to DMS and acrylate (not shown). The subsequent biological and photochemical oxidation of DMS to DMSO, sulfate and other products can occur within algae, bacteria, in the seawater and the atmosphere.
A picture describing some of the techniques they used to find out about DMSOP:
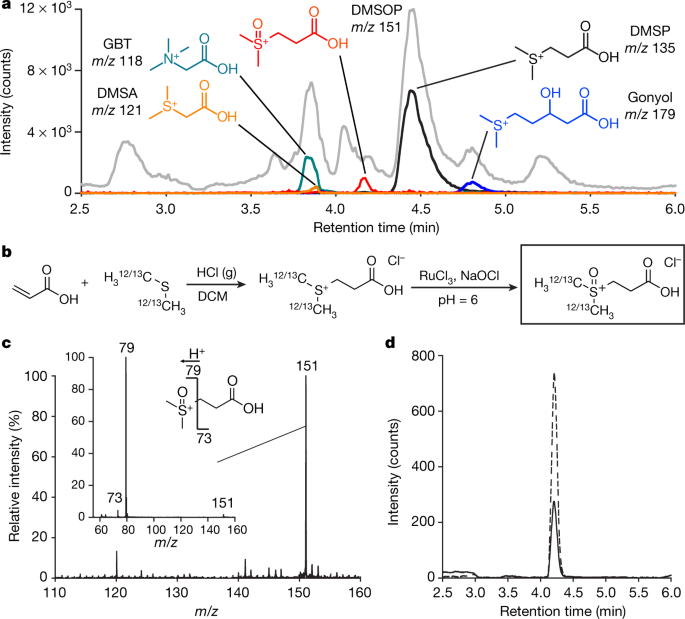
The caption:
a, Chromatographic profile of zwitterionic metabolites from a P. minimum culture, separated using ultra-high-pressure liquid chromatography (UHPLC) with detection by electrospray ionization mass spectrometry (ESI-MS). The total ion current is shown in grey. The metabolites glycine betaine (GBT, cyan), dimethylsulfonioacetate (DMSA, orange), DMSP (black) and gonyol (blue) were assigned according to a previous study16. The ion trace of DMSOP, red, is shown at a tenfold magnification. b, Synthesis of authentic (labelled) DMSOP. c, Mass spectrum and tandem mass spectrum (inset) of DMSOP with characteristic fragments. d, UHPLC profile monitoring m/z = 151 of an extract of P. parvum (solid line) and the same extract treated with synthetic DMSOP in roughly equal amounts (dashed line), the experiment was repeated three times with varying concentrations of synthetic DMSOP to confirm co-elution.
Biosynthetic pathways:
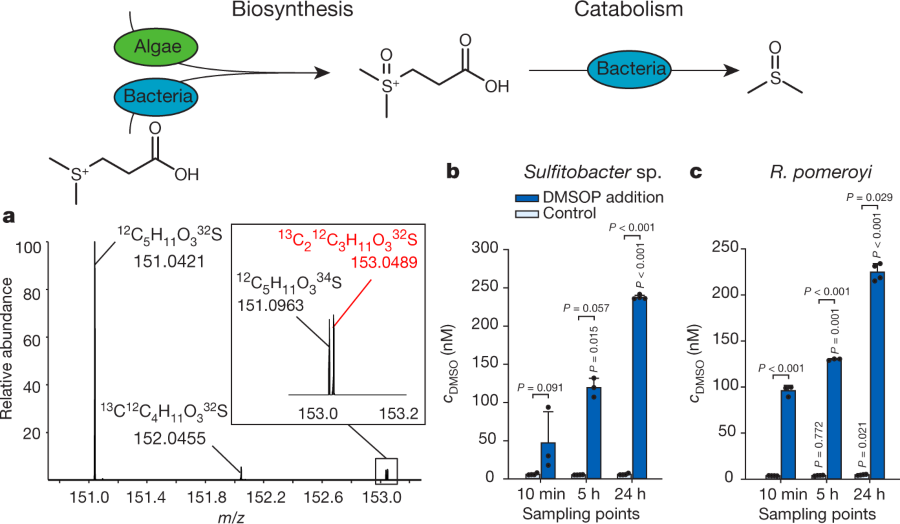
The caption:
a, High-resolution mass spectrum of DMSOP obtained from P. bermudensis incubated for 24 h with 13C2-labelled DMSP (Fig. 2). The peak labelled in red represents 13C2-labelled DMSOP, the natural DMSOP isotopes are shown in black (see also Extended Data Table 3). b, c, DMSO release (concentration (c) given as mean ± s.d.) of the bacteria Sulfitobacter sp. and R. pomeroyi incubated with 1 µM DMSOP. P values directly over bars indicate significant difference from t = 10 min of the same treatment, P values over brackets indicate significant difference between treatment and the control without DMSOP addition. n = 4 independent biological replicates for 24 h, n = 3 for 10 min and 5 h, for statistical details see Methods.
From the conclusion:
Our results demonstrate that the ubiquitous zwitterionic metabolite, DMSOP, contributes to the marine DMSO pool and may partly account for DMSO in marine algae13. In light of our findings, a functional role of DMSP as an oxygen acceptor is probable and could explain numerous observations of DMSP regulation under oxidative stress. Algal and bacterial DMSOP biosynthesis and its bacterial degradation to DMSO represent a previously undescribed pathway for DMSO production, extending our current paradigm of the marine sulfur cycle beyond the established biotic and photochemical pathways through DMS oxidation
Esoteric things like this are actually very important, particularly in light of the disturbance to the sulfur cycle represented by the rapidly increasing use of dangerous fossil fuels as we all wait, like Godot, for the grand "renewable energy" "revolution" that never comes.
I wish you a very pleasant weekend, and hope your Thanksgivings plans are proceeding nicely.


