Science
Related: About this forumRare Carbon, Nitrogen, and Oxygen Isotopes Appear Abundant in a Young Planetary Nebula.
The paper I'll discuss in this post is this one: Extreme 13C,15N and 17O isotopic enrichment in the young planetary nebula K4-47 (Ziurys et al Nature 564, 378–381 (2018))
Some background:
Carbon has two stable isotopes, 12C and 13C; Nitrogen has two as well, 14N and 15N; Oxygen has three, 16O, 17O, and 18O.
All of the atoms in the universe except for hydrogen, a portion of its helium and a fraction of just one of lithium’s isotopes, 7Li, have been created by nuclear reactions after the “big bang.” The majority of these reactions took place in stars, with some important exceptions: Lithium’s other isotope, 6Li practically all of the beryllium in the universe, and all of its boron. (Lithium, beryllium, and boron are not stable in stars, and all three are rapidly consumed in them; they all exist because of nuclear spallation reactions driven by cosmic rays in gaseous interstellar clouds.)
For the uninitiated, writing a nuclear reaction in the format 14N(n,p)14C means that a nucleon, in this case nitrogen’s isotope with a mass number of 14 is struck by a neutron (n) and as a consequence ejects a proton (p) to give a new nucleon, carbon’s radioactive isotope having a mass number of 14.
In the case of carbon, the nuclear reaction just described has been taking place in Earth’s atmosphere ever since that atmosphere formed with large amounts of nitrogen gas in it. Thus a third radioactive isotope of carbon occurs naturally from the 14N(n,p)14C reaction in the atmosphere as a result of the cosmic ray flux from deep space and protons flowing out of the sun. However, since carbon 14 is radioactive and since the number of radioactive decays depends proportionately on the amount of atoms that exist, it eventually reaches a point at which it is decaying as fast as it is formed. We call this “secular equilibirium.” The long term secular equilibrium at which carbon 14 is formed in the atmosphere at the same rate at which it is decaying is the basis of carbon dating. (This secular equilibrium has been disturbed by the input of 14C as a result of nuclear weapons testing.) Even with the injection of 14C as a result of nuclear testing, 14C remains nonetheless extremely rare and for most purposes other than dating, can be ignored, except perhaps by radiation paranoids.
In the case of all three elements mentioned at the outset, the isotopic distribution in the immediate area of our solar system is dominated in each case by a single isotope: On Earth Carbon is 98.9% 12C; Nitrogen is 99.6% 14N; Oxygen is 99.8% 16O. The abundances vary only very slightly in the sun.
An interesting nuclear aside: 14N has a very unusual property: It is the only known nuclide to be stable while having both an odd number of neutrons and an odd number of protons. No other such example is known. Note the correction to this statement by a clear thinking correspondent in the comments below.
In recent years, I've been rather entranced by the interesting properties of the fissionable actinide nitrides, in particular the mononitrides of uranium, neptunium and plutonium and their interesting and likely very useful properties, and in this sense I've been sort of wistful over the low abundance of 15N in natural nitrogen. In an operating nuclear reactor, with a high flux on neutrons - in the type of reactors I think the world needs, fast neutrons - the same nuclear reaction that takes place in the upper atmosphere, the 14N(n,p)14C reaction, takes place. Thus the inclusion of the common isotope of nitrogen in nitride nuclear fuels will result in the accumulation of radioactive carbon-14. In this case, given 14C’s long half-life, around 5,700 years – much longer than the lifetime of a nuclear fuel – secular equilibrium will not occur while the fuel is being used.
Personally this doesn't bother me, since it avoids the unnecessary expense (in my view) of isolating nitrogen’s rare isotope, 15N, and because carbon-14 has many interesting and important uses already. Carbon-14’s nuclear properties are also excellent for use in carbide fuels, inasmuch as it has a trivial neutron capture cross section compared to carbons two stable isotopes and, without reference to the crystal structure of actinide nitrides and mean free paths therein, and without reference to the scattering cross section of the nuclide (which I don't have readily available), it takes 15% more collisions (for C14 to moderate (slow down) fast neutrons from 1 MeV (the order of magnitude at which neutrons emerge during fission) to thermal (0.253 eV) neutrons than it takes for carbon’s common isotope 12C. (Cf, Stacey, Nuclear Reactor Physics, Wiley, 2001, page 31.) Thus 14C is a less effective moderator, and thus has superior properties in the "breed and burn" type reactors I favor, reactors designed to run for more than half a century without being refueled, reactors designed to run on uranium’s most common isotope, 238U rather than the rare isotope, 235U, currently utilized in most nuclear reactors today. Over the many centuries it would take to consume all of the 238 already mined and sometimes regarded as so called “nuclear waste,” access to industrial amounts of carbon-14 might well prove very desirable for the purposes of neutron efficiency.
Anyway...
The dominance of the major isotopes of carbon, nitrogen, and oxygen in our local solar system and in much of the universe is a function of stellar synthesis. Most stable stars destined to have lives measured in billions of years, including our sun, actually run on the CNO cycle, in which the nuclear fusion of hydrogen into helium takes place catalytically rather than directly.
Here's a picture showing the CNO cycle pathways:

Six of the nuclei in this diagram are stable, the aforementioned 12C, 13C, 14N, 15N, 16O, and 17O. However only 3 of them occur in other pathways, 12C, 14N, and 16O. When a main sequence star is very old and has consumed nearly all of its hydrogen, the only nuclei left to "burn" is 4He. The problem is that 4He has much higher binding energy than its nearest neighbors, including putative beryllium isotopes. Here is the binding energy curve for atomic nuclei, the higher points being the more stable with respect to the lower points:

Helium-4's anomalous stability prevents the formation of the putative isotope Beryllium-8. Observation of this isotope of beryllium is almost impossible since it's half-life is on the order of ten attoseconds, and it cannot actually form in stars. This is why 12C is a critical element in the pathway to the existence of all heavier elements. It forms from the simultaneous fusion of three helium atoms, and exists because it is more stable than helium-4. This is exactly what happens in dense stars when they have run low or out of hydrogen and only have helium left to burn. Carbon-12 can fuse with helium-4 to form oxygen-16. In addition, it can fuse with residual deuterium (2H) under these circumstances to form nitrogen-14. (However, in the helium burning phase deuterium, which forms from the p(p,γ )d reaction, where d is deuterium nuclei, is also relatively depleted.) Thus the formation of these isotopes is independent of the CNO cycle. As a result, it turns out that after hydrogen and helium which together account for 98% of the universe’s elemental mass, oxygen and carbon are respectively the third and fourth most common elements. These four elements comprise 99.5% of the elemental mass of the universe. All other elements, with nitrogen included, turn out to be minor impurities in the universe as a whole.
The minor isotopes in the CNO cycle are actually consumed in stars in this model, and to the extent that they exist, they simply raise the catalytic rate of hydrogen consumption.
All of this is the “understanding,” at least.
According to the authors of the paper cited at the outset, however, there seems to be other things going on in the universe, places where these reactions and their effects do not dominate. The authors are studying, at microwave and other frequencies, a planetary nebula that is estimated to be only 400-900 years old.
From the introductory text in the paper:
From the abstract of the paper, touching on the unusual nature of what the authors are seeing:
... These results suggest that nucleosynthesis of carbon, nitrogen and oxygen is not well understood and that the classification of certain stardust grains must be reconsidered.
They describe in the body of the paper the molecules they find that allow them to identify the isotopes, from the vibrational frequencies of their rotations which are effected by these, the frequencies being effected by the masses at the atoms of which they are constructed. (In the paper the techniques for the sensitive detection of these frequency variations is described.)
Figure 2:
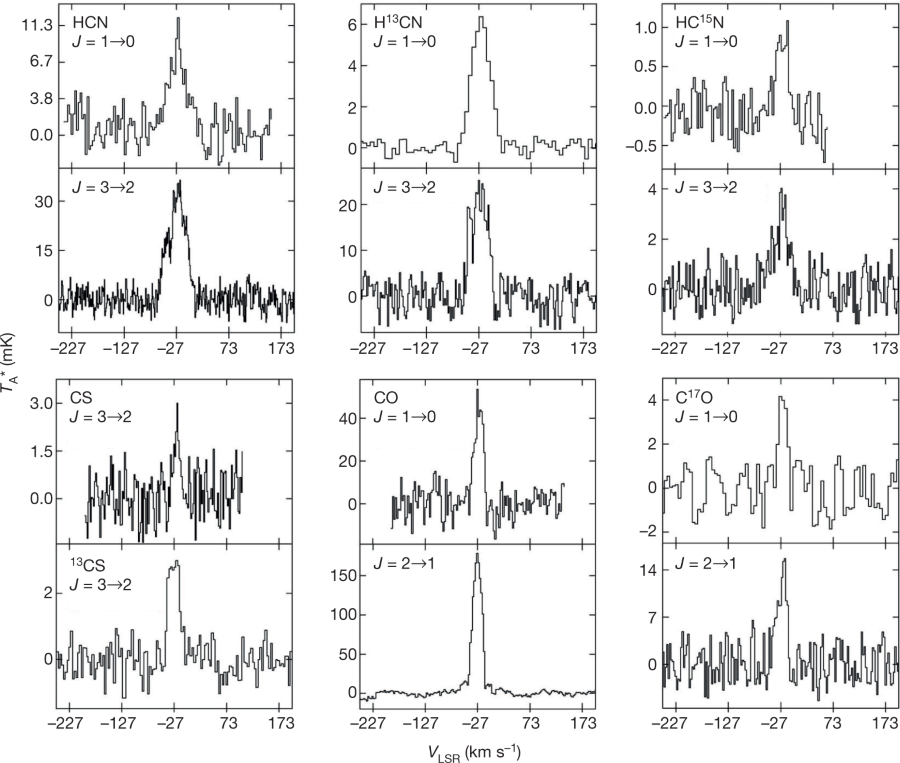
The caption:
Some further remarks:
A note on the rarity of this finding:
Aside from K4-47 and CK Vul, similarly low 12C/13C, 14N/15N and 16O/17O ratios have been found in presolar grains—small, 0.1–20-μm-sized particles extracted from meteorites28. These grains are known to predate the Solar System and originate in the circumstellar envelopes of stars that have long since died.
There is some discussion of the current theories of the origins of presolar grains, comprised largely of silicon carbide, thought to originate from "AGB" (Asymptotic giant branch) stars.
A graphic on this topic from the paper:
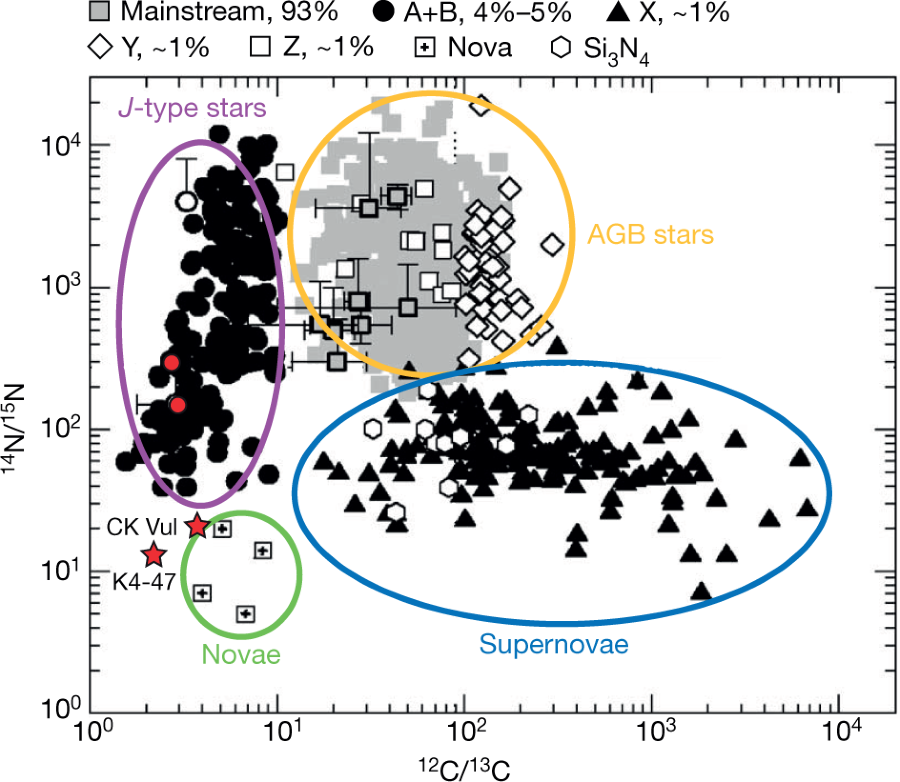
The caption:
Some final comments from the authors before technical discussions of methods:
50 years after the "Earth-rise" picture from Apollo 8 gave us a sense of our planetary fragility coupled with its magnificence, the rise of intellectually deficient, self absorbed fools, of which the asinine criminal Donald Trump is just one example, has threatened all that lives on that jewel planet first photographed from the orbit of the moon.
One feels the tragedy.
But the universe goes on, and for me, in this holiday season, it is good to feel its eternity, the beautiful facts that have no reason to be found other than that they are beautiful. In the grand scheme, I'm not sure we matter.
I wish you the best holiday season, and the peace that I found in this little paper, and that I wish that you, in your own place and own way, will similarly find.
Red Raider 85
(138 posts)Igel
(37,430 posts)eppur_se_muova
(41,299 posts)The former having three neutrons and three protons.
Then there's deuterium (one of each).
Aaaaand boron-10 (five of each).
***BUT*** nitrogen-14 is the heaviest such odd-odd nucleus, so yes, these things are rare. All other stable nuclei except helium-3 (that freak!) have a surplus of neutrons above the one-to-one ratio.
Still absorbing the rest of the article -- anything about O-17 tends to catch my eye, as it's kind of a sleeper in the NMR world (well, last I heard, anyway) IMHO. ![]()
NNadir
(37,534 posts)I was relying on my memory, and I'm an old man and can be excused on grounds of senility.
I know that 17O has a spin of 5/2, but I can't recall ever reading about NMR experiments involving it, although I'm sure it's been done.
I'm so old that when I was a kid, people had to enrich 13C to get an NMR signal from it, and now of course, with high field instruments it's rather routine with native isotopes, no enrichment necessary.
Of course 13C is more than 1%.
I imagine that a 17O experiment would involve a lot of acquisition time and a very high field instrument, but I don't know.
Amazing things are happening in NMR if I understand correctly. I went to a lecture by a Pfizer guy not too long ago where he seemed to have a pulse sequence to suppress solvent peaks so that he could follow, in real time, reaction progress by NMR.
I'm so old that I didn't think things like that were possible, but they are.
eppur_se_muova
(41,299 posts)*The only definite exceptions are 2D, 6Li, 10B, and 14N, mentioned in S. 12.50, which contain equal numbers of protons and neutrons.
-- Samuel Glasstone, Sourcebook on Atomic Energy, 3rd ed., Von Nostrand, 1967; pp.461-2. (A 1979 edition has been added.)
Emphasis added.
This book was one of the few good really books available in my high school library, and, as such, was shelved as a non-circulating reference work. What reading I did had to be accomplished in the short period in the mornings between arrival by bus and the homeroom bell, or during lunch periods, so I never got to read every part of it I would have liked, and in fact did not come across the statements above at the time (I paid much more attention to the descriptions of experimental apparatus at the time, IIRC). The curves referred to are energy-vs-Z curves derived from the liquid-drop model, with spin-spin effects included, and closely resemble some I came across *many* years later, in Povh. et al, Particles and Nuclei, Springer, 1995 (A 2015 edition is now available). These authors "cleaned up" the equations a bit, showing a neat quadratic equation in Z if one assumes A is constant. The neatness is a little deceptive, since one of the terms actually includes a dependency on spin-pairing, resulting in three different parabolas, one for odd/even - even/odd nuclei, which only beta-decay into each other, and one each for odd/odd and even/even nuclei, likewise, with a substantial energy drop from the former to the latter "built in". Unfortunately, these wonderful books are not on free Google Books yet, and the figures are hidden

(Diagonal sections through the plot would give constant-A parabolas, with the energy scale being inverted from the usual, with more stable nuclides having more positive values.)
The sharpness of curvature of the parabolas is of course determined by their second derivates, i.e. the quadratic coefficient. This actually has two parts, one inversely proportional to A, the other to the cube root of A, with the result that smaller values of A give narrower parabolas. Thus below A=15 the minima of the odd-odd curves can apparently fall below the nearest minimum-energy values of Z on the lower, even-even curve, but above A=15 both parabolas flatten out, and all odd-odd nuclei can beta-decay to more stable even-even species. (I haven't seen actual numerical plots, I'm just reasoning from algebra here.) Since positron decay produces products with larger total mass than the decaying nucleus, it is possible that some high-A nuclei may have *two* stable even-even nuclei to the right (increasing Z) side of the minimum. In fact, it appears that this only happens for Z=124 (124Sn, 124Te, and 124Xe are all stable).
All this has been worked out by the *real* experts -- see for example
https://en.wikipedia.org/wiki/Stable_nuclide
https://en.wikipedia.org/wiki/Valley_of_stability
but unfortunately I had to work some of this out for myself.
Above, I said "weird coincidence", but what really happens is that these discussions in E/E remind me of topics I had read in books which I no longer have access to, and so I add them to my wish list at Thrift Books, and every now and then one comes available for purchase at a reasonable price. Glasstone falls in that category, as did a wonderful book by Seaborg and several other titles in the Prentice-Hall "Foundations of Modern Chemistry" series (and I can't help but wonder what noble soul donated those volumes to the public library in that little tourist trap of a town. It was years before I realized how much I had benefited from that act of public-spirited generosity). So these little discussions have notably enriched the forced-involuntary-retirement phase of my life, and I appreciate your continued postings.
What all this has led to is an understanding -- embarassingly late in my career/education -- of the reasons some elements have multiple stable isotopes and a few with only moderately high Z have none (Tc and Pm), or one, which may even be a minor isotope (In, Re, Te). Ultimately, it turns out that there are only one or two stable nuclei for each mass number A, and since there are more than twice as many values of (alpha-decay stable) A than Z, of course a few values of Z will be missing, by statistically mandated accumulations of numerical coincidences. A chart of nuclear stability using Z and A axes -- as opposed to Z and N axes in all the published charts I have seen -- would make all this much more obvious, at the expense of a larger chart.
NNadir
(37,534 posts)They actually have 3 copies, two of which are in "Recap" - meaning they would need to be ordered from storage - and one that's still on the shelves.
The bibliographic information is here:
Format: Book
Language: English
Published/Created: Princeton, N.J., Van Nostrand [1967]
Εdition: 3d ed.
Description: vii, 883 p. illus., ports. 24 cm.
Notes: "Published under the auspices of the Division of Technical Information, United States Atomic Energy Commission."
Bibliographic references:
Includes bibliographies.
Subject(s):
Nuclear energy [Browse]
LCCN:
67029947 //r862
OCLC:
324616
Related name:
U.S. Atomic Energy Commission [Browse]
Other views: Staff view
I haven't gotten over to the Princeton library all that often recently; my career involves analytical medicinal chemistry which is better covered at Rutgers than at Princeton (since Princeton seems to be at war with Elsevier over Elsevier's pricing).
When I find myself there though, I'll definitely take a look, and perhaps scan it, since they have excellent scanners in the Princeton libraries which will scan books and documents to searchable PDFs. If a book is important enough, I will take an hour to scan it; I do it all the time.
As for what's in the book you graciously recommended:
It is sometimes the case that for two nucleons having the same mass number but a different Z, one proves to be unstable, with a very, very, very long half-life. This is the case, for example, for Xe-136 which was long thought to be stable but is in fact metastable with respect to Ba-136, with the half-life for double beta decay (through Cs-136) being on the order of 10^21 years.
I was actually relieved when it turned out that bismuth was unstable and radioactive, since it seemed to lack justice that the heaviest stable element had an odd Z.
I'm not entirely sure that there really are truly identical stable mass numbers, A, having two differing values of Z. It seems to me that it's quite possible that someday we'll discover that Ar-40 is unstable with respect to Ca-40 and so on...
Elements with exceptionally long half lives can play an important role in the utility of components of used nuclear fuels. Strontium is an element with four stable isotopes, 85, 86, 87, and 88. These mass numbers are arrayed on the ascending hump of the low mass fission products, as one can see in the chart in the OP for fission products from Pu-239. (The low mass hump is shifted slightly to the left for U-235, and U-233, but the high mass hump remains fixed with respect to nearly all of the common fissionable nuclei.) Since the ordinate is on a log scale, the differences in yield are larger than they seem at first glance. Sr-90 (with a half life of around 28) is a very valuable radioactive and prominent radioactive isotope among the fission products, since it establishes secular equilibrium with Y-90, and puts out significant heat as a pure beta emitter, making it useful for nuclear batteries. (The Soviets actually utilized this isotope in this way for remote arctic signals.) However the specific thermal output is a function of the radiochemical purity of this isotope, which in theory could be contaminated with the stable isotopes. The extremely long half life of Rb-87 - so long that it occurs naturally and is a significant part of "natural" rubidium, and represents an important geologic clock - prevents the accumulation of Sr-87, and although the fission yield is lower, Kr-85 can be separated with relatively fast reprocessing, blocking the accumulation of Sr-85. (Sr-86 and Sr-88 remain a problem however.)
When I consider your situation and your obvious love of knowledge for its own sake, I especially appreciate how fortunate I am to live in New Jersey and to have access to these great libraries. I can accumulate reading materials much faster than I can possibly read them, and feel that with some exceptions - mostly the nuclear journals (and my son's university has those) - I can read almost anything I want to read, pretty much find out anything about which humanity already knows. This is unbelievably good fortune, and I'm always amazed that people around here don't seem to use these resources with the same passion that I do. I pay to use Princeton's libraries, but there is almost nothing I've bought in my life that's quite as valuable. Even better, my public library has an interlibrary loan program that allows me to order books in the rare case that neither Princeton nor Rutgers has them
It has allowed me to learn and become expert in subjects for which I have no formal training. .
I realize that not many people read or care about what I write here - some do, but not many - but writing here helps me clarify in my mind these fascinating things to which I expose myself, and it's an extra benefit if someone actually enjoys what I write, understanding that some people reject and even hate what I write.
On a personal level, if I gave my sons anything worthwhile, that thing might be that they are both autodidacts, having learned that practice from me. It's my one saving grace as a father, inspiring those boys to learn things independent of any formality.
They are getting excellent formal educations, but they aren't limited by them. My youngest son came home from his recent semester college having learned to play some very sophisticated jazz piano pieces, as well as sophisticated classical piano pieces, as well as having learned to speak credible (I'm told) Russian and Chinese, this while pursuing an engineering degree, having never had a piano lesson or having taken a course in Chinese or Russian. My oldest son, who is about to get a BFA in design and painting has spent the Christmas break writing code for AI programs to recognize and report colors. He's never had a computer course, nor for that matter a calculus course, but he's taught himself matrix algebra and can hold a fairly good conversation on subjects in calculus. (His high school rebellion was to inform me that he wasn't going to take any more than minimal math courses to graduate, since he was planning to be an artist. He now has decided that I was right to try - even if I failed - to force the issue.)
There are so many things we can teach ourselves if we try. The internet helps of course, but libraries are still the hope of the world, I think, and are one of the least appreciated great resources by which we can help ourselves to be able to help save what is left to be saved and can still be saved. (We're running out of time.) I wasted a lot of my life before I found this out.
Thanks again. I wish you a happy New Year filled with new knowledge.
NNadir
(37,534 posts)I had about two hours in the library yesterday, since it was raining and my wife had no projects for me, was busy herself, and my sons were visiting friends.
I found the Glasstone book and was very surprised at how wonderful it was.
My kid recently insisted on buying Pauling's General Chemistry even though I have a copy here of my own. He wanted a copy that was his, because even though the book is decades old, it still is written in a way that's clear and inspiring, and gives a feel for bonding that one just doesn't see elsewhere, coming from a pioneer.
It's timeless.
Another book that's timeless, that will still be worth reading after decades have passed is McQuarrie's Quantum Chemistry.
The Nuclear Energy Sourcebook has that quality.
My life might well have been different if my parents, back in 1967 - neither of whom finished high school and one of whom never went to high school - had known that such a book existed. As it was, the only academic book we owned in 1967 was a Bible Commentary from a Theology School, although I recall that year my parents really struggled to find the money to buy the Encyclopedia Britannica; certainly a worthwhile purchase for students before the internet.
The Nuclear Energy Sourcebook, from what I gathered as I peeked at the text while scanning it, surveys the development of atomic theory right from ancient times through Dalton, Boltzman, Einstein, Bohr and is written clearly and certainly at a level that a precocious high school student could easily read with not too much strain, while covering the topic without compromise. After more than half a century the book still gives, from what I can see, an excellent feel. (The brief preface is written with a reproduced signature of Glenn Seaborg, then serving as the Chairman of the Atomic Energy Commission.)
Very, very, very beautifully done.
I really can't thank you enough for pointing it out. If I ever have grandchildren, and live long enough to see them, I'm going to point it out, and if their dad turns out to be my youngest son, he can also give the Pauling's book to read, because again, they are timeless and still true.
defacto7
(14,162 posts)and for your mind stretching exercises that, in this world of diminishing returns, gives a bit of hope and wonder.
Have a great winter holiday season.
NNadir
(37,534 posts)I wish you a peaceful and happy holiday season as well.
Mind stretching is a wonderful sport, even better than skiing, and unlike skiing, which may decline with the coming non-existence of snow, it's available to everyone, with no limits. The more we learn, the more we'll be able to help in saving what is left to be saved of the world.
I'm not all that smart, particularly compared to my two sons, but to compensate for my failings, I read a lot. (One of the greatest joy of my life is that my boys learned to be autodidacts from me: The stuff they've taught themselves entirely independently of their course work, rather stuns me.)
I appreciate greatly that someone, anyone, reads this stuff and enjoys it as much as I enjoy writing about it. Thanks again for your support.
