Science
Related: About this forumA Detailed Thermodynamic Accounting of Yet Another Wind to Hydrogen Scheme.
The paper from the primary scientific literature I'll discuss in this post is this one: Sustainable Hydrogen Production from Offshore Marine Renewable Farms: Techno-Energetic Insight on Seawater Electrolysis Technologies (Rafael d’Amore-Domenech*† and Teresa J. Leo† ACS Sustainable Chem. Eng., 2019, 7 (9), pp 8006–8022)
Here's a cartoon from the paper:

It's um, a cartoon.
Recently I wrote a post about another paper published in the primary scientific literature over in the E&E forum, along the same lines, also featuring a cartoon that also implied that the scheme therein was workable, although the authors concluded at the end that it wouldn't work. In that scheme, hydrogen was - as it must be in any workable scheme for its use in energy storage - a captive intermediate.
That post is here:
A Detailed Thermodynamic Accounting of a Route to Obtaining World Motor Fuels from Solar and Wind.
The current paper focuses on the hydrogen and not the downstream motor fuel described in that post OMEn ethers.
In the chemical industry, we say that a molecule is a "captive intermediate" if it can only be used in a chemical plant setting, and is not, or perhaps better said, should not be, sold as a commodity. Despite much hype by assholes like Amory Lovins, who once claimed that hydrogen HYPERcars would go mainstream, hydrogen is not and never will be a consumer fuel, despite the fact that its combustion product is pure water. However, hydrogen is already industrially a "captive" intermediate on a huge scale for many economically important processes, notably the production of gasoline. Almost all of the hydrogen on this planet, much better than 95% is made by the reforming of dangerous fossil fuels, mostly dangerous natural gas, but also, dangerous coal. Probably the use of captive hydrogen that has the most impact on all human lives, as opposed to the "upper crust" of humanity which is more concerned with tooling around in cars than having access to even primitive sanitation, is for the production of ammonia, without which at least half, if not more than half, of the world's population would be required, quite literally, to starve to death.
In modern liberalism, as opposed to the liberalism into which I developed as a young man more than 4 decades ago, we are more interested, I worry, in electric cars than we are in people who starve to death, or who are enslaved as children to make, um, electric cars.
My son likes to challenge me, so for Christmas a few years back, he bought me this book: The Half Has Never Been Told. It is a very painful book to read. If you can read this book without weeping, you're one cold sonofabitch or daughterofabitch as the case may be. The thesis of the book is that modern American wealth derives from its history as a slave holding nation, that the capital infrastructure that allowed for its historic industrialization and the wealth that has survived to this day, was only possible because of the involvement of all Americans, North and South, in the slave driven cotton industry. (The mutual involvement of North and South in human slavery in North America was the thesis of Abraham Lincoln's Second Inaugural Address as well.)
If you believe that modern wealth in the United States and other first world countries is not involved in modern human slavery, including the slavery of children, I suggest you pull that cell phone out of your pocket and look at it, or turn the damned portable computer over a stare at what's in the battery compartment, and ask yourself if you are lying to yourself.
In my experience as an old man, it is very easy for people to lie to themselves, easier in fact than not lying to themselves. I know this from personal experience.
Today, whether we choose to face it or not, pretty much the wealth of the whole world is involved in human slavery, since there are more cell phones and batteries than human beings and they are involved in virtually every country's economy.
Facts matter.
Anyway, that small matter out of the way, let's talk about wind power and hydrogen:
Some years ago, when I was writing for the E&E group, going back to a time before it was a group, there was a lot of hype being written there about ten houses on an obscure island that is part of Norway called Utsira, where there was an executed plan to run the island all wind derived hydrogen. Since water is thermodynamically more stable than a mixture of hydrogen and oxygen - which is why such a mixture is subject to explosions like the ones that destroyed the nuclear reactors at Fukushima - any hydrogen on Earth is stored energy and not primary energy. One would have to be as dumb as Amory Lovins to not know that, and apparently a lot of people are, since one hears hydrogen often described as a "clean fuel." This is pure garbage. Again, almost all of the hydrogen on Earth is made from dangerous natural gas, and description of dangerous natural gas as a "clean fuel" is pure marketing, and its acceptance by many people is simply another example of people's willingness to lie to themselves.
But what about hydrogen from wind energy. Wouldn't that be "green" and "sustainable?" That would depend on whether or not wind energy is "green" and "sustainable."
Beware of lying to yourself.
The Utsira wind plant was supposed to be a demonstration of how wind energy would save the world.
It hasn't; it won't.
The Utsira project has been reduced to "lessons learned." If you'd like to read all about "lessons learned" at Utsira, here's a link to a place you can do so: The wind/hydrogen demonstration system at Utsira in Norway: Evaluation of system performance using operational data and updated hydrogen energy system modeling tools (International Journal of Hydrogen Energy Volume 35, Issue 5, March 2010, Pages 1841-1852). The full paper has a wonderful photograph of the hydrogen plant on Utsira, which also shows the base of the huge steel tower for the wind turbine.
Here it is:

If you think huge steel towers are "green," beware of lying to yourself.
You can just make out some little red dots in the background of the picture. Those are items of clothing on human beings, presumably some of the human beings who lived in the ten houses this contraption was supposed to power.
If you do not have access to a scientific library, or if the one to which you have access is at war with Elsevier, I will be happy to share some text from this paper about "lessons learned" at Utsira, the demonstration plant which was supposed to save the world but, um, didn't:
The bold and italics are mine.
Hydrogen represents, again, energy storage. As I repeat all the time, being a scientist who is trying to prevent people from lying to themselves - a quixotic enterprise at best - storing energy wastes energy, a consequence of the irrefutable 2nd law of thermodynamics. Generating hydrogen from grid electricity is so thermodynamically absurd as to be, well, disgusting, particularly to "prove," using ten houses on an island, that wind power "could" save the world. (The issue of whether making hydrogen from grid power is thermodynamically (or environmentally) disgusting may be less of a concern, however, in Norway, since Norway, an offshore oil and gas drilling hellhole, produces most of its electricity from hydro power. Hydroelectricity is not really sustainable, particularly with the acceleration of climate change, but it is less noxious than other forms of so called "renewable energy," in my opinion).
My hypothesis when arguing about the value of the Utsira plant back in the old days at E&E was that it would fail to provide 100% of the power to ten homes on a remote Island. The "lesson I learned," if not anyone else, was that my hypothesis was borne out by experiment. It failed.
From 2007 to 2017, the world as a whole "invested" 1 trillion, 31.5 billion dollars (US) in wind energy.
This information is here, in the UNEP Frankfurt School Report, issued each year: Global Trends In Renewable Energy Investment, 2018
1.0315 trillion dollars is more than the GDP of all but the top 15 nations of the world. It is more than twice the gross domestic product of that offshore oil and gas drilling hellhole Norway, three times the gross domestic product of that offshore oil and gas drilling hellhole Denmark, and more than the gross domestic product of Indonesia, a nation with 261,000,000 human beings living it it.
If the intended result of this investment was to address climate change, the results of this very, very, very expensive experiment are written in the planetary atmosphere:
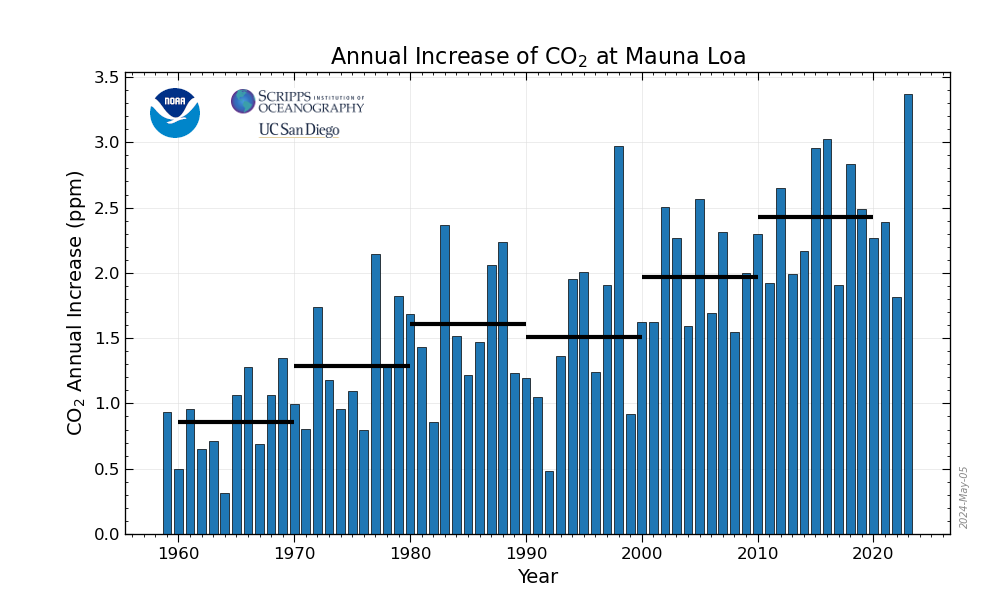
I follow carbon dioxide concentrations at Mauna Loa fairly closely, in considerable detail. It is possible that the growth in carbon dioxide concentrations in 2019 will exceed the growth observed in 2018, and possibly even the growth in 2016, the first year ever recorded in which the rise in these concentrations 3.00 ppm in a single year.
So much for the idea that "investing" in the energy in the atmosphere, wind energy, will save the atmosphere...
But the idea that we can make wind work - if the work we seek to accomplish is to address climate change and not to require more coal to make steel - if only we could store the energy it collects at random refuses to die, even among scientists.
Sometimes we see the word "scientists" used, particular in lay renderings, to convey authority, truth, practicality or many other positive attributes. Sentences and titles along these lines begin with verbs like "say" or "plan" or "find" or "think" as in "Scientists say...," and "Scientists think...," and "Scientists discover...," and so on. Probably it is the case that in many cases the authority is valid; it is certainly true that the Nobel Laureate Glenn Seaborg knew more about nuclear energy than the fool Amory Lovins who calls himself a "scientist" even though there is little evidence that Amory Lovins has a clue about science. When Glenn Seaborg spoke about nuclear energy he spoke with authority. However, when the racist Nobel Laureate William Schockley spoke about the relationship between genetics and the vague (and effectively undefined) concept of intelligence, he was merely a crank. If, on the the other hand, Shockley spoke about semiconductors he had authority, even if as a human being he was less than worthless.
I have personally in my lifetime have almost certainly spoken with tens of thousands of people possessing Ph.D's in various areas of science. Many of them are quite impressive. Some are true polymaths, experts on any subject one raises. On the other hand, some of them are not. There are some people who hold advanced degrees in sciences, even from good schools, who, when you meet them and talk with them, you wonder how they ever learned to operate a can opener, never mind write an acceptable thesis. There are many people who are very good at their area of expertise and completely clueless on all other topics. Even if one is an expert on a particular topic, one can draw conclusions that are wrong. The co-discoverer along with Franklin and Crick of the geometry of DNA, Nobel Laureate James Watson, is an expert in genetics, obviously. That he is also recently been discovered to have been a racist does not imply that the racist genetic views that he (and Schockley) hold or held are valid. Enrico Fermi claimed to have discovered Neptunium and was awarded the Nobel Prize for doing so, except that what he actually discovered (but didn't recognize as such) was nuclear fission, which was recognized some years after his "discovery," by Lise Meitner, reviewing, not Fermi's work, but the results of her one time partner Otto Hahn. Now Fermi was, in my opinion, the greatest scientist to bridge theory and experiment since Newton, or possibly Archimedes and so that he was wrong about Neptunium does not imply that he was a bad scientist, but it does imply that even the greatest scientists can make mistakes in interpretation. There are scores of things discovered and accomplished by Fermi that were worthy of Nobel Prizes, including the practical discovery of how to extract usable energy from uranium.
However, given that even Fermi could be mistaken, I think it wise to advise one to use a little critical thinking when encountering a sentence written by a lay person that begins with "Scientists say..."
My son and I have a little tired running joke which begins with reference to my palilalia in which I repeat the question, "Did I ever tell you what Einstein said about thermodynamics?" to which my son replies, "I think you did. Did you say that Einstein said that thermodynamics was the one branch of science he never expected to be over turned?"
"That's right."
Einstein was often right, but sometimes he was also wrong. I don’t think he was wrong about thermodynamics though.
This brings me to the paper I promised to discuss. From the introduction to ACS Sustainable Chem. Eng., 2019, 7 (9), pp 8006–8022):
Now, in my opinion anyway, if someone says, "Scientists..." (in this case Drs. d’Amore-Domenech and Leo) "...say that 'the world is undergoing an environmental and energetic crisis due to the intensive use of fossil fuels.1 As a result, people of heavily populated areas are starting to develop respiratory afflictions.2 The vast emission of greenhouse effect gases is subjecting cold regions to great stress, quickly destroying ecosystems and menacing wildlife...'" that person would be, in my opinion, relaying an indisputable truth, with the possible exception of the use of the word "starting," since people have been dying from respiratory afflictions from dangerous fossil fuel waste for many scores of decades and are not "starting" to do so.
If on the other hand if one says..."Scientists say 'In this sense, there are two possible pathways for the mitigation of this problem [the environmental energetic crisis]: diminishing our economic activity or changing our energy use toward a more sustainable one: Regarding the latter, it is necessary to increase the renewable energy proportion in our mix...'" one would not be stating an irrefutable truth.
In fact there is evidence that the opposite is true: The expenditure of an amount of resources greater than the annual gross domestic product of a nation containing almost 4% of the world's population (Indonesia) on one form of putative "renewable energy," specifically wind energy, has done nothing to mitigate the environmental problem of dangerous fossil fuels. In fact, in spite of this vast expenditure, things are getting worse, not better.
Facts matter.
The scientists continue to say:
Scientists say, " hydrogen solutions are postulated to be more permanent."
Speaking only myself, I certainly hope not, not that I intend to demean "scientists," in general although I'm quite willing to demean non-scientists saying the same thing, like, for example, the so called "Chief 'Scientist'" of the "Rocky Mountain Institute" who say the same thing about hydrogen cars that the real scientists say here.
Reference 14 in the paper can be found here: Global Automotive Executive Surveys. I have not accessed the report on what global automotive executives think, nor do I have any interest in doing so. I couldn't care less what they think about hydrogen cars or anything else.
My comments aside, there is a lot of good information in this paper, and it's well worth a read, especially if it your habit to lie to yourself, something almost all of us do at one time or another. The truth can be painful, but it is the truth, whether we avoid it or not.
The authors give reasons for focusing on the use of seawater to generate hydrogen by electrolysis, and to be clear, I also regard seawater as the best ultimate source of sustainability, albeit with a very different, diametrically different, approach to its utilization.
The authors' representation of why they think seawater is important:
The emphasis on the word "could" is mine. My whole damned adult life - and I'm hardly young - I've been hearing about what so called "renewable energy" could do but very little about what it is doing, at least in ways that matter. In terms of energy (as opposed to the dishonestly stated representations hyping peak capacity while ignoring capacity utilization depending on the weather) so called renewable energy represented by wind, solar, geothermal and tidal combined in this century "grew" at a rate that was, in this century, that was only 6% as fast as the rate at which the use of dangerous fossil fuels grew, by 8.12 exajoules as compared to 135.18 exajoules for dangerous fossil fuels. Overall, energy demand grew by 165.83 exajoules from 2000 to 2017, 2017 being the last full year for which data is available.
Overall, dangerous fossil fuels provided, in 2017, 472.77 exajoules as compared to 10.63 exajoules for wind, solar, geothermal and tidal combined.
2018 Edition of the World Energy Outlook Table 1.1 Page 38 (I have converted MTOE in the original table to the SI unit exajoules in this text.)
Thus there is no experimental evidence at all that wind power is capable of replacing dangerous fossil fuels. Despite this vast record of abysmal failure, there is an impetus to evaluate how we might get hydrogen from wind power using seawater if we could build vast wind facilities capable of producing 472 exajoules of energy in a single year. This is like betting the farm on what kind of townhouse you could buy in Manhattan if you won the lottery, but frankly, you won't win the lottery, even if you buy millions of lottery tickets from the proceeds of selling the farm.
Quoth the authors:
One has recently begun to see these sort of graphics in papers on theoretical approaches to energy suggesting combinatorial optimization of the theoretical feasibility of approaches to energy systems.

The caption:
Presumably in this graphic, the combinatorial "outlook" for marine so called "renewable energies" is represented by comparing the areas between the figures dark blue lines defined by the length of vectors running from the center of the hexagon in the direction of each of the vertices. I'm personally not sure that these diagrams mean anything at all, since it's clear that the weighting by which one defines the length of the vectors is subjective. To wit: Is the "levelized cost" of a wind turbine trashing the benthic ecosystem of the continental shelf in New Jersey, the same as it might be in a wind farm trashing the benthic wilderness near Dutch Harbor, Alaska? Is the weighting of cost the same off the coast of New Jersey the same as it is in Alaska? There is simply no description of the calculations used to generate these figures - perhaps they are available in some or all of the references 31-36 - but it's of no matter, let's take the author's word for it, that of all possible efforts to derive energy from oceanic systems, the most available and lowest cost is offshore wind turbines. It's easy to make this assumption because, even though offshore wind energy has had no measurable effect on the growth in the use of dangerous fossil fuels, it clearly produces the most energy of all of the forms listed in the cartoon/graphic, although all forms, including the one producing "the most," are trivial forms of energy on this planet.
All this out of the way, let's get to the interesting part.
The following graphic shows the type of electrolyzers evaluated in the paper:

The caption:
Before looking at some of the details of the scheme, the authors make a point that definitely has thermodynamic consequences of the energy efficiency of the overall process.
None of these options, as we are seeing carbon dioxide concentrations as dangerous fossil fuel waste at 415 ppm, roughly 25 ppm than what we saw just ten years ago, when we were already 20 or 30 years into caterwauling about how wind energy would save the day, are commercial. If they were, however, they'd be fairly intense energy drains. If the energy were to be available only from so called "renewable energy" of course, this would reduce the amount of so called "renewable energy" available for electrolysis.
Almost certainly the most energetically expensive option is liquefaction. The critical temperature of hydrogen is only 33 K above absolute zero (-239.95C) and the critical pressure is roughly 1,300 kPa, about 13 times atmospheric pressure. Above this temperature and below this pressure, hydrogen cannot be liquefied. The authors state that pressurized shipment of the gas is probably the ideal way to get the gas to land, since, they state, all of Europe's existing offshore wind farms are within 110 km of the shoreline, and they note that the shipment of dangerous natural gas is cheaper than shipping liquefied dangerous natural gas over such short distances. Of course, under conditions where there was actually enough wind energy to actually store any of it, this might not be true, since orders of magnitude more wind turbines would be required, since right now, after decades of wild cheering, wind energy remains a trivial form of energy in Europe and everywhere else.
Now let's turn to the electrolysis technologies. On the first the author's write:
• Production of hydrogen and oxygen
• Production of chlorine, hydrogen, and alkalis
• Production of hydrogen and hypochlorites...
In noting that these processes are industrial already for purified NaCl or KCl, the authors state that where they are so, hydrogen is a by product and that the goal of running them is actually to produce chlorine for chlorinated chemicals (some of which represent serious environmental problems) and or KOH or NaOH caustics, and bleach (sodium or potassium hypochlorite) and to a lesser extent perchlorates for the explosives and rocket industries, also environmentally problematic compounds.
They continue:



Note that for KCl(aq), eq 3 changes the NaCl(aq) for KCl(aq), and the NaOH(aq) for KOH(aq).
The production of chlorine at sea, the authors note, is likely only to be an environmental problem, particularly on any scale that was meaningful. In addition the concentration of salts in commercial electrolyzers is considerably higher, roughly 25% as compared to roughly 3.5% in seawater meaning large energy losses owing to the higher electrical resistance in a seawater based electrolysis unit. Then the other salts in seawater, sulfate, calcium, magnesium would almost certainly lead to scaling on the electrodes, requiring periodic acid washes and finally, the electrodes they have in mind are iridium impregnated titanium dioxide, and very expensive. Commercial chlorine electrolyzers have, historically, utilized mercury electrodes, and historically (and to a lesser extent today) bleach has been a major source of environmental mercury pollution, although considerably smaller than the two larger forms, the combustion (or coking for steel) of coal, and medical waste.
Thus conclude the authors:
Nevertheless, they carry the process through their discussion.
The next electrolysis concept discussed in the paper as equivalent were covered as two separate types in my last major post in the E&E forum on this site, the one on "wind energy to motor fuels" which I linked above. These two, here treated together, are alkaline electrolysis and PEM electrolysis, the former being hydrolysis of relatively pure sodium or potassium hydroxide solutions, the latter being electrolysis using proton exchange membranes, abbreviated as "PEM."
They combine these two forms under the rubric of "Low Temperature Electrolysis." They write:
...The electrolyte can be either NaOH or KOH, as stated in the Introduction. In any case, KOH is usually preferred since it presents better specific ion conductivity.19 The redox reactions in alkaline electrolyzers go as follows:



This type of electrolysis technologies operates between 60 and 90 °C.72 Their architecture is quite simple, and can also operate under pressure, up to 42 bar, which allows energy savings when output hydrogen is needed under compressed state.73 Their operating efficiencies pivot around a value of 85% (1.7−1.8 V), referred to the higher heating value (HHV) of hydrogen, at current densities that typically range from 100 to 300 mA/cm2, although recently, current densities from 1000 to 1500 mA/cm2 have been reported for that same efficiency.7
As one drawback listed, the authors mention the possible leakage of hydroxide solutions into the ocean, although personally I wouldn't worry too much about this, since the ocean is a carbonate/borate buffered system increasingly undergoing acidification, in part because all of our faith in so called renewable energy hasn't panned out, isn't panning out, and won't pan out.
On the subject of PEM electrolyzers the authors write:



In comparison to alkaline electrolyzers, PEM technology presents a great reduction in size and weight, not only because of the type of architecture but also, because they present greater current densities for the same operating efficiencies. Specifically, they exhibit around 1 A/cm2 for about 85% efficiency (1.7−1.8 V),82 referred to the HHV of hydrogen, at temperatures ranging from 60 to 80 °
The authors list several drawbacks, including cost, although they expect these costs will fall, and the possible poisoning of the membrane with ions like magnesium and calcium. They completely ignore the question of Nalfion, which is a fluorocarbon polymer, the degradants of which, including the degradants of teflon, also a fluorocarbon polymer, represent a huge environmental challenge for future generations, not that we give a rat's ass about future generations as our day to day behavior illustrates.
The authors note that these two "low temperature" electrolytic cells that they combine here both depend on access to fresh water. This means that the putative hydrogen plants are required to desalinate water. Although they note that reverse osmosis (RO) membranes are more energetically efficient for this purpose, they consider that the water so obtained is not as pure as water obtained by distillation, which can involve two approaches, one simple distillation at atmospheric pressure, by definition here at sea level, and therefore at 100°C, or reduced pressure involving the use of compressors. They give reasons for choosing the latter, wisely I think. Historically, this type of device, a distillation apparatus, has suffered from corrosion problems in seawater desalination, and in fact, most of the world's desalination plants are RO units, although I believe that modern materials science may offer an avenue to revive distillation as a strategy that has the advantage, at least for systems that are cleaner and more sustainable than wind energy, for example, nuclear energy, for the recovery of waste heat. Here, by contrast, all of the heat is obtained by wasting electricity, although, as we will see, the authors represent this waste heat as minor compared to the energy consumed by electrolysis itself, which, in fact, does generate some waste heat as ohmic losses.
At this point, it is worthwhile to examine another paper on the subject of the electrolysis of seawater that has received no comment from the authors since they focus only on hydrogen. Heather Willauer of the US Navy Research Laboratory has developed a device that electrochemically splits seawater into acidic and basic fractions. The concentration of carbon dioxide in seawater is much higher than it is in air, which is a good thing, because if the oceans were not effective extraction devices for carbon dioxide from the atmosphere we would be talking in terms of thousands of parts per million of the dangerous fossil fuel waste carbon dioxide in the atmosphere rather than the 415 ppm we have recently reached because of our misplaced faith in things like wind turbines to address the problem. In Willauer's device in the acidic fraction this carbon dioxide is driven off and recovered as a pure gas, where the basic portion is available for use alkaline electrolysis to produce hydrogen and oxygen. The hydrogen can then be utilized to hydrogenate the recovered carbon dioxide to make fuels, in Willauer's scheme, jet fuel for aircraft carriers.
One of many descriptions of this process available in various places in the literature, and convenient for me to pull up quickly is this one: Extraction of Carbon Dioxide and Hydrogen from Seawater by an Electrochemical Acidification Cell Part III: Scaled-up Mobile Unit Studies
This process, represents, if optimized, an approach to reversing climate change, but has not received as much attention as perhaps it should, possibly because the immediate application is a military application. Of course, it is the case that many systems originally developed for military purposes have proved enormously important in general use, examples including the computer, the GPS, the nuclear power plant, etc...
Whatever...
The authors of this paper discuss a third category of electrolysis driven by marine wind farms is a high temperature approach, the solid oxide fuel cell run in reverse as an electrolysis device. They write:



The architecture of SOECs can be either tubular or planar. Tubular architecture shows very good resistance to thermal cycling; however, it is more difficult to benefit from cost reduction under mass manufacturing. For this reason, planar designs are preferred, although they are less robust regarding thermal fatigue.19...
...Solid oxide electrolysis cells typically operate around 500 mA/cm2, and they present efficiencies of around 95% (1.3 V) referred to the lower heating value (LHV) of hydrogen. Their reported durability goes up to 10 000 h at continuous operation.86 However, with shutdowns, their durability is shortened due to the induced thermal fatigue. The main problem of this technology in a marine renewable context lies in the unlikeliness of finding an external source of high temperature in the whereabouts of the offshore renewable farm.87 Thus, the production of superheated vapor and the heat management of the electrolyzer would be done at the expense of the electricity generated by the farm. Therefore, the overall efficiency of the process, that is thermal and electrical, would be penalized.
A point to be made as an aside: 700°C is well above the critical temperature of water, 373°C, and if the pressure is also above the critical pressure, which is about 1,300 kPa, the water will be in a supercritical state where, more or less, salts are insoluble. Although the authors have correctly, I think, rejected this option for wind turbines, although the carry consideration of the case through the paper, managed correctly, raising seawater to supercritical temperatures is in my opinion, a technology that humanity should consider to survive, and a technology that will actually help clean the ocean of the serious problem of micro and macro plastic pollution, as well as indirectly clean the atmosphere of carbon dioxide. Wind power won't cut it for these purposes, but nuclear energy might do this quite well. The Thermodynamic Equation of State, TEOS-10, of seawater is a much discussed topic, typically of interest to oceanographers and geologists, but well worth consideration by nuclear and other engineers.
To return to the paper currently under discussion, a word on some of the discussions of device lifetimes seems appropriate. The 100,000 hour lifetime figure for alkaline electrolyzers amounts to about 10 years of life, which is actually less than the relatively short life times of wind turbines.
In various places at various on the internet, by appeal to the Danish Energy Agencies comprehensive database of Danish wind turbines, I have calculated the average life time of decommissioned wind turbines in that offshore oil and gas hellhole of a nation. Most recently, I did so here:
Average Lifetime of Danish Wind Turbines, as of February 2018.
I decided to play with the Excel functions to update the data.
I'm amused to report that the average lifetime of failed wind turbines has in fact, increased. It is now 17 years and 240 days. The longest lived turbine made it to 35 years and 240 days, a 22 kw unit commissioned on January 9 1981 and decommissioned on September 6, 2016.
Of the 3,232 decommissioned turbines, 3 others made it to 35 years, and 14 more than 30 years.
Of course, there are some that never operated at all, and 157 that operated ten or less years.
My son, the materials science engineering student, is home for a week before heading to Europe and then to Oak Ridge National Laboratory for his summer internship, and we've been watching engineering television including "Air Disasters" on the Smithsonian channel, and "Engineering Catastrophes" on the Science Channel. I believe it's episode 11 where the failure of the edges of highly engineered wind turbine blades is discussed, as a cause of the performance degradation of these steel monstrosities intended to make all of our wild spaces on land and on sea into industrial parks. Noted in the program is the fact that when we set out on this so called "renewable energy" adventure, many people assumed that wind turbines would be "maintenance free." It was also assumed that wind turbines would address climate change. However, again, after a more than trillion dollar investment, there is no evidence that this is the case. The climate is deteriorating more rapidly than it was before this expenditure and we have now crossed the 415 ppm mark for concentrations of carbon dioxide in the atmosphere for the first time in human history.
The replacement of devices incurs not only an economic cost, but an environmental cost as well. This is an important thing to keep in the back of one's mind when one is considering what is and what is not "clean" and/or "green."
But let's return to this wonderful paper on wind powered hydrogen which fans of the Utsira pilot represented as a technolgy which would save the world:
The paper launches into a discussion of relatively routine thermodynamic concepts. I won't cover all of it here but point to some highlights.
There's a nice discussion of the work of compression, which, as we have seen, plays a role in the efficiency of these systems.

where pin and pout are the pressure at the inlet and at the outlet of the set of compressors, respectively. To remove all crossover gas sourced from the anodic side of the cells, passive autocatalytic recombiners are used. This provides an effective means for purifying the hydrogen stream, through the reaction of the crossover anodic gas with hydrogen.90 The plant is considered to be 10 m above the sea level. From that point onward all the plant is considered to be at the same height, with ideal flow, without pressure drops. Therefore, kinetic and potential terms of the total enthalpies are neglected. The power consumption estimation is assessed by appliying the first principle of Thermodynamics on every device:

where Q̇ is the heat transferred to the device, which is zero for adiabatic devices, Ẇ is the power supplied to the device and...
...and so on.
A discussion of mass balance and a review of some electrochemical concepts:

The theoretic molar flow rate is defined by the Faraday law:

where I is the current, z is the ratio of exchanged electrons per mole of the gaseous output in the redox reaction (4 for oxygen and 2 for hydrogen), and F is the Faraday constant 96485.3399 C/mole e−.
The following equations define the molar flow rates in the cathode output, where hydrogen is the main component:



where psat(T) is the saturation pressure of water and p is the pressure of the stream at that point.
The following equations define the molar flow rates in the anode output when oxygen is produced:



where psat(T) is the saturation pressure of water and p is the pressure of the stream at that point.
...and so on.
Here are the diagrams of the hypothetical plants that the authors utilize in their modeling calculations.

The caption:

The caption:

The caption:
They consider recovery of the waste heat in the latter case, said heat flows being discussed widely in the scientific literature under the general rubric of "process intensification." I wrote some bits about process intensification in my last major post in the E&E forum linked above, and for convenience, should anyone be remotely interested, linked once again.
A Detailed Thermodynamic Accounting of a Route to Obtaining World Motor Fuels from Solar and Wind.
It's a little disingenuous in my opinion to be discussing process intensification to address a technology for which the point of the entire discussion is storing energy because the technology provides random intermittent power that may not be available when it is needed and may be available in excess when it is not needed. Process intensification is a very good idea, but process intensification assumes continuous processes, because otherwise, particularly for heat exchange, a system which is off will come to thermal equilibrium with the environment. It will thus require reheating of all the components, including the exchangers to operate which suggests that it might actually waste more energy than it recovers.
Nevertheless they write:
The profound environmental problem with so called "renewable energy" revolves largely around its high mass intensity. I noted, with reference to an article in Nature Geosciences in the previous thread this about the mass intensity of wind energy were it dedicated to making enough energy just to make enough motor fuels to displace petroleum.
The translates into 53 billion tons of steel, 5.2 billion tons of aluminum, and 673 million tons of copper. The production of steel involves coal, the current process for the production of aluminum utilizes petroleum coke electrodes (which are oxidized), and copper involves intense heat and the release of copious quantities of sulfur oxides.
Renewable? Really? Whither the ores for this pixilated process?
What I did not note was the coal intensity of steel. The world coal association has a wonderful page on the subject of making steel. It is here: Uses for coal, how steel is produced. It has interesting factoids like this:
The coking process consists of heating coking coal to around 1000-1100ºC in the absence of oxygen to drive off the volatile compounds (pyrolysis). This process results in a hard porous material - coke. Coke is produced in a coke battery, which is composed of many coke ovens stacked in rows into which coal is loaded. The coking process takes place over long periods of time between 12-36 hours in the coke ovens. Once pushed out of the vessel the hot coke is then quenched with either water or air to cool it before storage or is transferred directly to the blast furnace for use in iron making.
...and...
This means that to produce 53 billion tons of steel would require 37 billion tons of coal, most of which would be oxidized to CO2, thus producing about 135 billion tons of carbon dioxide. Current annual emissions of carbon dioxide from all dangerous fossil fuels is roughly one fourth this amount, roughly 35 billion tons per year and rising despite the world wide rote enthusiasm for so called "renewable energy."
The addition of process intensification equipment that would only be marginally effective when utilized in systems with capacity utilization well below 50% would further add to this already unsustainable material burden.
Think. Do not lie to yourself. Think.
Anyway here are some graphics showing the author's results.

The caption:
The numbered points on the abscissa in this graphic refer to the numbered streams in the graphic schematics for each of the three processes. Note that for the direct hydrolysis of seawater, one element of the process stream is chlorine gas. Elsewhere in the paper on this issue the authors write with admirable honesty:
More graphic conclusions:

The caption:
The overall energy requirements and rejected heat to produce 1 kg of compressed hydrogen gas:

The caption:
From this data we can see that in terms of energy efficiency, the high temperature SOFC plant is the most efficient requiring a little over 250MJ/kg of compressed H2.
The energy density of plutonium is 80.3 million MJ/kg. Thus 250 MJ represents 3.1 micrograms of plutonium, an amount which just might be visible to the naked eye if your eyesight is excellent.
Plutonium fueled plants, by the way, are very much subject to process intensification, although few in modern times actually are. This is because plutonium fueled plants have a remarkable record of running at 100% capacity utilization or close to it. They do not require redundant plants.
We should ask ourselves a basic question about wind energy's economic costs - costs that I consider to be relatively small when compared to much larger environmental cost - the question being "what is the cost of the redundancies required to make it work?"
In 2017, the last year for which we have data, as I often remark, wind, solar, tidal and geothermal energy combined generated 10.63 exajoules of energy out of 584.98 exajoules generated and consumed by humanity.
In terms of average continuous power, this amounts to the output of about 337 1000MWe power plants, 1000MWe being a rather typical output of the world's reportedly 62,500 power plants
Of course the average power of so called "renewable energy" plants has nothing to do with their performance since often they are producing much more than 337,000 MW, generating all kinds of breathless posts here and elsewhere of how "renewable energy" briefly produced "x" percent of the power in country, city or town "such and such." At other times these plants produce vastly less energy, sometimes less than zero. This means that the plants always require a redundancy, whether it involves the hydrogen examined here, or as is actually the case pretty much everywhere, a redundant dangerous fossil fueled power plant.
The fact is that were the "renewable energy" fantasy actually come to pass - it won't, but let's play pretend - we would be placing our energy supplies in the hands of the weather, and would be assuming the absence of wind droughts, massive floods, endless supplies of materials, massive dust generating fires, and "all the natural shocks..." that infrastructure..."is heir to." We would be doing so at precisely the time that the world's weather system is seriously destabilized, as we're seeing pretty much every damned day because the renewable energy fantasy did not work and is not working. It also will not work.
The amount of plutonium required to produce 337,000 MW of power would amount to about 4 grams per second, or about 132.4 metric tons per year.
For way of contrast, here's a report on the engineering of a wind turbine: High-Strength Steel Tower for Wind Turbines
According to this engineering report, from data on page 102, the weight of a single wind turbine’s wind turbine, the tower alone, is 146 tons. A single wind turbine requires more mass than the plutonium fuel that could make all of these monstrosities existing or planned unnecessary.
Now if you think it is more difficult to contain 132 tons of a very high density metal fission products, or the waste of billions of tons of steel by products, including coal waste since steel always requires coal to make, your knowledge of engineering is extremely low, which doesn't make you unusual of course, but if you are concerned about waste and you think that 132 tons of fission products are more difficult to contain forever than tens of billions of tons of steel (and aluminum waste products, you are lying to yourself, whether you know it or not. (For the record, I don’t believe in burying fission products, since all of them are useful critical materials, but that’s another subject.)
Used nuclear fuel in the United States amounts to, at a first order of magnitude, around 75,000 tons. A basic rule of thumb is that used nuclear fuel contains about 1% reactor grade plutonium, suggesting that we can recover about 750 tons of plutonium from it. (Roughly 95% is unreacted uranium and the rest is useful fission products.) There's a lot of foolishness about how many "nuclear bombs" one could make using 750 tons of plutonium as if processing it to do this involved nothing more than a trip to Home Depot and 15 kg of readily plutonium to make a nuclear weapons. The critical mass of plutonium depends of the geometry in which it is placed and the materials surrounding it, but let's as a rule of thumb, say that 20 kg could be utilized to start a controlled chain reaction. This suggests that we could start 37,500 small chain reactions, and if the material surrounding it is once through or depleted uranium, we could sustain these reactions for decades without ever mining a damned energy material, no oil, no coal, no gas, in fact no uranium, since we have more than a million tons of the stuff already isolated.
It won't happen probably, but not because it's impossible, but because humanity is too stupid, to wrapped in fear and ignorance to do it.
So let's return to the paper to see what the promise of wind powered hydrogen is, despite the failure of the wind turbine at Utsira to power ten homes.
From the authors conclusions:
An energetic assessment for offshore production of pressurized hydrogen at 350 bar, using electricity sourcing from marine renewable energies has been performed on three different electrolysis technologies: direct seawater electrolysis, low-temperature electrolysis, and high-temperature electrolysis.
Results have revealed that direct seawater electrolysis is completely unfit for green hydrogen production at sea, mainly because of the very high specific energy needed to produce hydrogen. Specifically, it requires about 160% more energy than the low-temperature electrolysis, but also due to the environmental impact that it implies.
For most cases in marine renewable scenarios, when plant dynamics come into play, low-temperature technologies seem to be the most practical. This is because low-temperature electrolysis adapts better to the power variation inherent to most renewables. In this regard, the specific energy to produce hydrogen at 350 bar with respect to high-temperature electrolysis in marine offshore conditions at steady state is only a 13% higher. Regarding the specific energy consumption, the best technology operating at steady state conditions is the high-temperature electrolysis. Such technology could be unrivaled whenever an external heat source of high temperature was available nearby.
Large-scale production of marine green hydrogen is not currently profitable because its resulting price without state aid is not competitive with other production methods. This is mainly due to the high costs of electricity sourcing from marine renewables. Such costs are expected to decrease in the following years, perhaps to the point where this source of green hydrogen becomes competitive. For grid-connected offshore renewable farms, small-scale production of marine green hydrogen could be competitive at present, since in most cases curtailed electricity often benefits from better prices.
The bold here is mine.
The conclusion comes with the de rigueur repeat of the usual mantra that is always repeated about so called "renewable energy" that someday it will be competitive, especially if we ignore the external costs of environmental damage and the internal costs of requiring redundancy, something that is routinely done with zero ethical integrity, because ignoring such costs is a way of lying to oneself and/or to everyone else, it doesn't matter which.
There is nothing "green" about this putative hydrogen about which we are supposed to be happy.
I've been listening to happy talk about so called "renewable energy" my whole damned adult life, and I'm not young. Everyone's been happily waiting, entirely unworried as to whether or not they are lying to themselves, said lying coming at the expense of all living things and all future generations of human beings.
This is not right. This is wrong, in every way, but most importantly morally wrong.
If you look at the cartoon at the outset of this post, you'd think it's easy, a done deal. The devil in the detail is that it won't work.
We have now seen, for the first time in human history, measurements showing that the concentration of carbon dioxide in this planet's atmosphere has reached 415 ppm, while we wait...while we wait...for "costs expected to decrease."
History will not forgive us, nor should it.
I trust you're having a pleasant weekend.
Turbineguy
(39,915 posts)As someone who has run saltwater distillers for years, I think that separating hydrogen from seawater by electrolysis would be nearly impossible as a practical matter, although it might be useful as an inefficient way to mine gold or obtain other minerals.
Also I think having stored hydrogen in cars akin to using dynamite to propel a car saying, "oh, we have a method of controlling the reaction so that it will be nice and slow!"
NNadir
(37,557 posts)...and highly impure sources; one thing they're going to have to do is to go through our waste dumps to recover materials they need.
One of our biggest waste dumps is the oceans.
There are three critical materials that are readily available from seawater; one is fresh water, obviously, the second is carbon in the form of carbon dioxide, biomass and plastic waste, and the third most important in my mind, is uranium.
It is possible I think to utilize reduced pressure distillation techniques, alluded to in the paper discussed in the OP, to purify water in an energy efficient manner, particularly by process intensification, that is by putting what is now considered "waste heat" to use. The intakes can be adjusted to recover uranium, although humanity will actually not really require uranium for centuries in a plutonium cycle. (There are, however, good reasons to recover uranium anyway from sources having NORM (naturally occurring radioactive materials) and mine tailings and the like.)
Other elements that you mention, for example gold, may be recoverable depending on price and demand. The price wasn't high enough in 1919 when Fritz Haber tried to do this to "save" Germany.
If we are going to utilize biomass, plastic waste, and the various forms of carbonates in the oceans, this is going to require supercritical temperatures, which is very different than anything we're doing now. I consider that supercritical fluid technology will be an absolutely essential technology if we have any hope of sustaining the future, not just supercritical water, but supercritical carbon dioxide, and other supercritical materials like current day curiosities like DME.
The recovery of elements from mining tailings, waste dumps (including the atmosphere and the oceans), will require the overcoming of the entropy all previous generations have left for subsequent generations. This of course will require huge amounts of energy.
The use of supercritical water would give a relatively clean way to desalinate and simultaneously concentrate the minerals in seawater. I expect that some of these recovered materials, particularly brine, will represent disposal problems in their own right. This is already a problem where desalination is widely used. I have never worked with TEOS-10; since I lack the time and quite honestly the computer skills to do so, and so I'm not sure whether the thermodynamic equations are validated to the supercritical regions and the resultant phase systems. It's possible that they have been, since supercritical seawater is discussed in some papers I've seen relating to oceanic volcanoes and other geothermal systems. I do hope that I can convince my son to consider these equations of state among others.
I will say this, at high temperatures such as those obtained in supercritical water, many processes are known for thermochemical waster (and carbon dioxide) splitting, and the production of hydrogen as a captive intermediate and not as a consumer fuel, as you say, is relatively straight forward from seawater. I also think that as a side product of seawater processing, excess electricity might be utilized, particularly where it required as "spinning reserve," the solid oxide fuel cell described in this paper seems quite attractive to me to produce hydrogen. I do believe these can be adapted to seawater, while conceding that current technologies do not cut it where seawater is concerned, as the authors of this "wind to hydrogen" scheme plainly confess.
There is really only one option to provide the energy required for all this, uranium, and to a lesser extent, thorium and/or deuterium and tritium if they ever actually make nuclear fusion work. One thing is clear is that nuclear fusion will not be available in any time frame to address the on going and worsening crisis.
Mersky
(5,340 posts)Wow, this is a big post! I find it very readable, and I don't mind the scrolling.
I definitely come away from this with a new perspective on how in the hell we're gonna possibly meet the demand for electricity with the current piecemeal scheme of a relatively few more wind turbines and solar panels compared to the shocking number of power plants globally (62,500!!!). Rather appreciate your emphasis that redundant power plants are needed for existing 'renewables'.
And, I've noted that plutonium power plants don't need any, eh, backup power generation. I'll go seek more info about the ins and outs of nuclear efficiencies of cost and waste/storage/reprocessing (although, any recommendations are appreciated). If it's just a question of engineering, the value may become evident and relatable. I will ponder this.
After another read through, I might be able to follow new developments in hydrogen generation. And, I didn't know how much I needed a refresher on the particulars of coking coal. Basically, I'm floored anew by the scale of the problem of efficiently using materials for steel consuming wind turbines, etc.
I've bookmarked this, and will check out your detailed thermodynamic acc...
NNadir
(37,557 posts)Let me say this:
The Pavlovian association of nuclear energy with issues of waste is rather strange, because if you ask someone to show a case where so called "nuclear waste" has actually killed someone, they really can't do it, or, if they informed enough to do so (as there have been a few cases of things like fatal criticality accidents in processing used nuclear fuel), they cannot compare this to the deaths associated with dangerous fossil fuel wastes, which have killed tens of millions of people in the last decade alone.
I will take these concerns about "wastes" seriously when people can demonstrate that they can do simple comparisons between things differing by 6 or 7 orders of magnitude.
More than 7 million people die each year from dangerous fossil fuel waste and dangerous biomass combustion waste:
Here is the most recent full report from the Global Burden of Disease Report, a survey of all causes of death and disability from environmental and lifestyle risks: Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015 (Lancet 2016; 388: 1659–724) One can easily locate in this open sourced document compiled by an international consortium of medical and scientific professionals how many people die from causes related to air pollution, particulates, ozone, etc.
As for cost, if one really wants to think seriously on this topic, one should consider what the cost - economic if not moral - of climate change is.
There is actually no such thing, to my mind, as "nuclear waste," because I consider all of the elements in used nuclear fuels to be materials that will prove incredibly valuable.
I have written on these topics extensively here and elsewhere.
The best summation I think, albeit somewhat dated, I have ever written on my philosophy on these issues is here:
Current Energy Demand; Ethical Energy Demand; Depleted Uranium and the Centuries to Come.
Thank you very much, again, for your kind words, but even more for thinking. I live for that, people thinking because of what I've written.
Mersky
(5,340 posts)Have been reading news and thoughts at DU for many many years. Decided to get in the conversation here to keep from randomly screaming at head-in-the-sand folks I encounter on the regular here in central Texas.
Will keep working my way through your posts and recommended links. Honestly, reading that long piece of yours turned into a respite from the daily onslaught of political news. This, even as the consequence of humanity not getting real about carbon emissions rang thru loud and clear. Ya might see me chime in now and again with with questions, some of which may be more dumb than I realize.
(For whatever it's worth, I've been an atheist for three decades now, and science fills the spiritual space and time that church going would require. I have an underutilized degree in economics, roughly remember my calculus, and generally can digest topics of chemistry/physics as a know-it-all daughterofanengineer. Basically, I'm a nerd.)
NNadir
(37,557 posts)I have made it a point in my life to work as hard as I can to be the dumbest person in the room as often as is possible, and now whenever I go to a talk that is beyond me, I make a point of struggling to find a question to ask, especially if the topic is completely new to me.
It's a good way to learn.
Almost 100% of speakers will be patient and accepting.
I recall one talk I went to where I guy asked a speaker on the genetics of the ocean something along the lines of, "did space aliens build the pyramids," and I admired the patience and respect that the speaker, Kay Bidle of Rutgers Department of Oceanography, gave the questioner.
Prof. Kay Bidle, Rutgers University: The Invisible World of Marine Microbes: How Earth’s Smallest Living Things Have the Biggest Impact on How Our Ocean Works
My sons still laugh at the face (as an audience member) I made when the question was asked, but Dr. Bidle was very gracious, and I learned a little bit about patience from his manner of response every bit as important as what I learned all about ocean microbes in the body of the talk itself.
It was a beautiful thing.
I'm sure you won't ask me about space aliens and the pyramids, but if you do, don't worry about what I think. Questions are good, all of them.
After all these years, now that I'm an old man, when I go to certain kinds of regular meetings, people expect a question from me, since I've gotten better at it, and I'm very proud of that.
You can't be good at anything you don't practice.
Thanks again for your kind words and again, for thinking. Politics are important, but in a Democracy we cannot guide our best leaders unless we know what we're talking about. The time of Trump will pass; but the problems of the planet will still need to be addressed and the more we know, the better we'll do.
