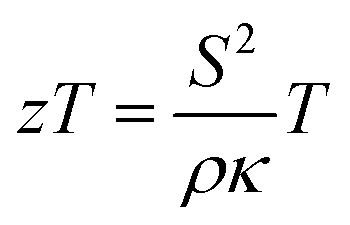Vacancy-Doped Neodymium Telluride for Thermoelectric Applications
The paper I'll discuss in this post is this one: Synthesis and Characterization of Vacancy-Doped Neodymium Telluride for Thermoelectric Applications (Bux et al, Chem. Mater. 2019, 31, 12, 4460-4468)
Recently, according to my son who is interning there, the Oak Ridge National Lab resumed the production of plutonium-238, the isotope that has powered many interplanetary space missions, the most famous recent example being the wonderful New Horizons mission that visited the Pluto/Charon systems (Plutonium is named for Pluto, which was as the time of the discovery of the element, considered a planet) as well as the Ultima Thule Kuiper belt object.
The plutonium-238 RTG's have functioned beautifully, some for multiple decades.
The introduction to the paper discusses one such mission, the Voyager mission, still growing strong, but describes its limitations:
Radioisotope thermoelectric generators (RTGs) have been a key enabling technology for the National Aeronautics and Space Administration (NASA) to power deep-space exploration vehicles. These generators utilize thermoelectric materials to convert heat from the spontaneous decay of a radioisotope into electrical energy, providing power to the spacecraft. Because thermoelectrics are solid-state devices, the nature of RTGs has allowed them to demonstrate long-term reliability, as evidenced by the Voyager I and II missions, which have been continuously operating for over 40 years.(1,2) Although RTGs are robust and highly dependable, state-of-the-art RTGs utilize heritage thermoelectric materials (Si–Ge alloys, PbTe, and Te–Ag–Ge–Sb) which exhibit only modest thermal-to-electrical beginning-of-life conversion efficiencies of approximately 6.5% at the system level.(3) These conversion efficiencies are limited by the low average values of the dimensionless figures of merit (zT) of the state-of-the-art materials across their operating temperature ranges.(3−6) zT is defined as zT = S^(2) T/ρκ, and high-performance materials will possess a large Seebeck coefficient (S), low electrical resistivity (ρ ), and low thermal conductivity (κ ). Furthermore, because the efficiency of a RTG is dependent on the temperature gradient across the thermoelectric material, ΔT, materials with higher operating temperatures are of interest as well. Identification of new materials with high zT’s and with high melting points is desirable to potentially increase the specific power of RTGs (W/kg), which could allow for more scientific instrumentation on a spacecraft while also reducing the amount of radioisotope heat-source fuel required for a specific power output.(7,8)
Because of the limitations of the editor at DU for mathematics, let me post graphic equations for the dimensionless figure of merit from another paper (Synder and Synder, Figure of merit ZT of a thermoelectric device defined from materials properties,
Energy Environ. Sci., 2017,10, 2280-2283 :
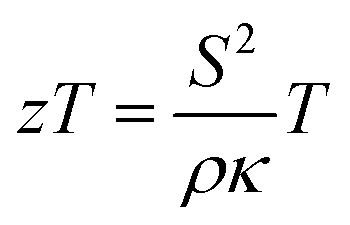
where S is the Seebeck coefficient, ρ is the electrical resistivity, κ is the thermal conductivity and T is the absolute temperature of the material at the point in question.5 The figure of merit zT(T) is, in general, a temperature dependent material property derived from temperature dependent material properties S(T ), ρ

T), and κ (T). An efficient thermoelectric generator, however, must operate across a finite temperature difference ΔT = Th − Tc so that these material properties will change from the hot to the cold end.

The maximum efficiency η of a thermoelectric generation device is also traditionally characterized by the thermoelectric device figure of merit, ZT whereimage file: c7ee02007d-t4.tif (2) The overall maximum efficiency of the generator is limited by the Carnot factor, ΔT/Th, and the reduced efficiency that depends on ZT, Th and Tc. Eqn (2) is typically derived assuming the thermoelectric materials properties S, ρ, and κ are constant with respect to temperature, exactly matched n-type and p-type legs and 1-dimensional heat flow with no other losses.2 It is only in this case of constant S, ρ, and κ that the material figure of merit zT (at T = (Th + Tc)/2) and the device figure of merit ZT (evaluated between Th and Tc) are the same.
"zT" here is
not a product, but is rather a traditional representational symbol of "figure of merit."
As is the case with straight up Carnot efficiency, it can be seen here that the distance between the high temperature and low temperature dictates the efficiency of the system. Therefore thermoelectric systems that work at high temperatures are to be preferred.
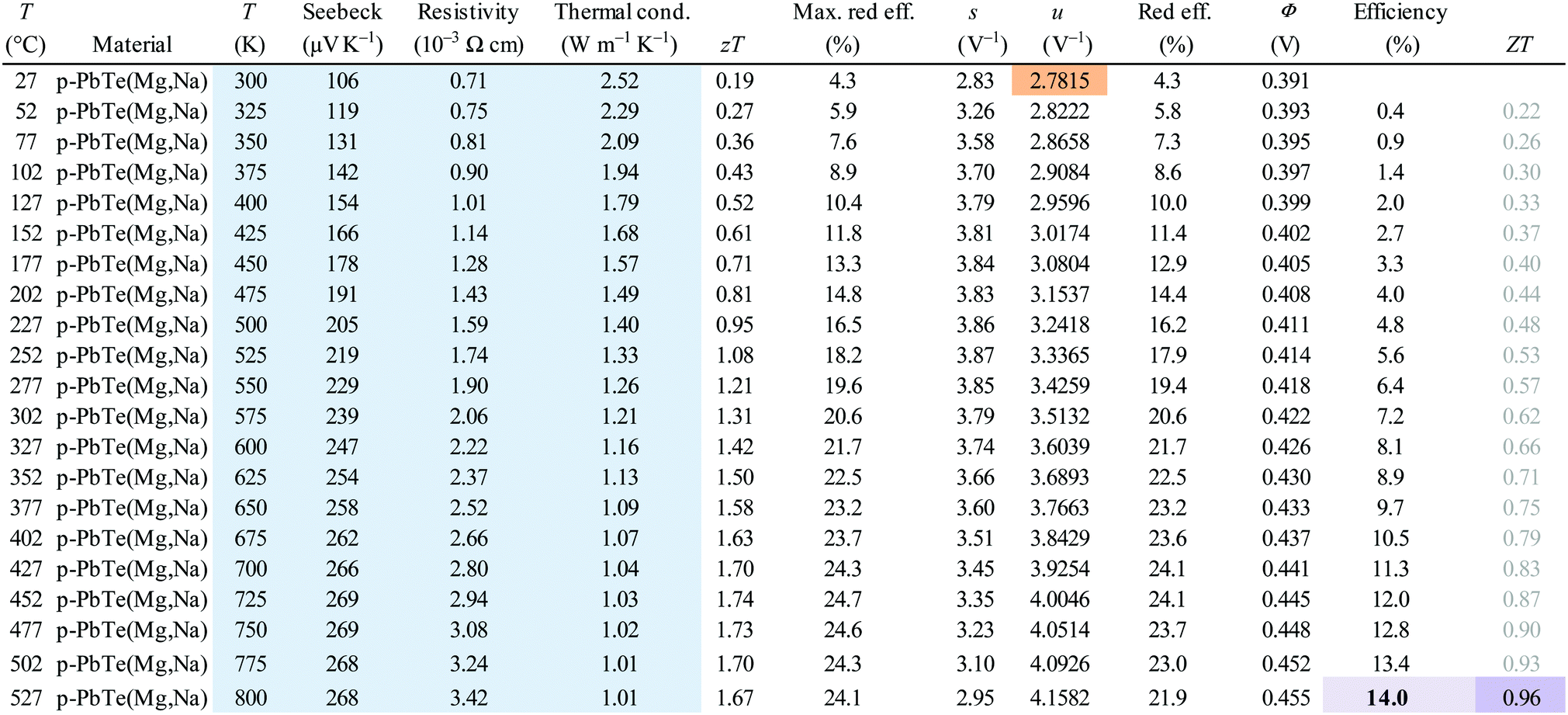
A table from the Synder paper gives a feel for the relationship between efficiency and the figure of merit:
Returning to the original paper under discussion, the authors have worked with a new material, neodymium telluride, to achieve relatively high Seeback coefficients.
The material is being compared to and designed around another lanthanide telluride, lanthanum telluride. This material has a figure of merit of 1.1 at 1275K.
Some technical talk around this topic:
Lanthanum telluride (La3–xTe4) has been identified as a high-temperature, n-type thermoelectric material.(3,8,9) La3–xTe4 adopts the Th3P4 structure type (I4̅3d) with 28 atoms per unit cell, and this structure allows for La3+ vacancy concentrations of up to x = 0.33.(8,9) The carrier concentration of La3–xTe4 is controlled by these La3+ vacancies, as each lanthanum atom contributes 3 electrons, and each tellurium atom accepts 2 electrons. With no vacancies (x = 0, La3Te4), there is one free electron per formula unit and metallic behavior is observed. With the maximum vacancy concentration (x = 0.33, La2.67Te4), there are no free electrons present and it behaves as an insulator. Furthermore, La3–xTe4 possesses an intrinsically low lattice thermal conductivity (κL) resulting from the complex Th3P4 structure, phonon point-defect scattering from vacancies, and electron–phonon scattering at more metallic compositions. The combination of these favorable properties results in a high zT of 1.1 at 1273 K with an optimized stoichiometry of x = 0.26 (La2.74Te4).(8) The excellent electronic properties of La3–xTe4 stem from the large Seebeck coefficient that results from heavy conduction bands.(10) Computational modeling has shown that the electronic density of states (DOS) of La3–xTe4 is controlled by the La states in the conduction band, whereas the valence band states are dominated by the Te atoms.(10−12) In the conduction band, a peak in the DOS near the Fermi level enables La3–xTe4 to maintain a high Seebeck coefficient at high carrier concentrations (n ≈ 0.9 × 1021 when x = 0.26). Modifications of the conduction band states have been performed using divalent substitutions (Ca and Yb) on the La sites, but these were found to have little impact on zT.(9,12) Inspection of the conduction band states shows that they are primarily composed of the La 5d states, with some contribution coming from the empty La 4f states. The limited contribution of the 4f states is a result of La having no 4f electrons. Recently, praseodymium telluride (Pr3–xTe4), another rare-earth (RE) telluride with the Th3P4 structure type, was investigated, and through band structure calculations, it was found that the introduction of the three 4f electrons of Pr resulted in a significant shift of the peak in the DOS closer to the conduction band edge when compared to La3–xTe4.(13) As a result, at equivalent vacancy concentrations, Pr3–xTe4 exhibited a 25% improvement in the Seebeck coefficient over La3–xTe4. This increase, coupled with a lower thermal conductivity, resulted in a zT = 1.7 at 1200 K for Pr3–xTe4. There exist many other Th3P4 structure type compounds.
Some pictures from the text:

Figure 1. (A) Electronic DOS for Nd3Te4 and La3Te4, band structure diagrams for (B) La3Te4 and (C) Nd3Te4. (D) Predicted Seebeck coefficients as a function of carrier concentration for Nd3Te4 and La3Te4 calculated using density functional theory.

Figure 2. (A) X-ray diffraction pattern on a ground compact of Nd2.78Te4 including profile fit, difference, and residuals obtained by performing Rietveld refinement. The measured pattern corresponds well with the Th3P4 structure type and does not indicate the presence of any secondary phases. The two broad peaks near 22° and 26° 2θ are residual background from a Kapton film used to protect the air-sensitive samples. (B) BSE SEM image of a representative sample (Nd2.84Te4). The uniformity of the image contrast reflects the homogeneity of the sample. Dark areas are indicative of sample porosity.

Figure 3. (A) X-ray diffraction patterns for pellets of Nd3–xTe4 at various compositions. The range of compositions Nd3Te4 through Nd2.78Te4 agrees with a phase-pure cubic Th3P4 structure type. An oxide-contaminated pattern is given here as an example, with the largest non-overlapping oxide peak labeled. The Nd2.67Te4 sample adopts the orthorhombic Th2S3 structure type. (B) XRD pattern for Nd2.67Te4 before and after a 4-day anneal at 1373 K, showing the expected phase transition from the Th2S3 to the Th3P4 structure type. (C) XRD pattern for Nd3Te4 before and after a 7-day anneal at 1173 K, showing no phase transition. The largest non-overlapping oxide peak is labeled with an “O”.

Figure 4. (A) WDS calculated vs Hall measured carrier concentrations for Nd3–xTe4 samples, showing excellent agreement between calculated and measured values. (B) Temperature-dependent resistivity for Nd3–xTe4 samples compared to La3–xTe4.(13) Nd3–xTe4 showed more resistive character relative to La3–xTe4 at equivalent vacancy concentrations. (C) Plot of carrier mobility vs carrier concentration at 600 K for Nd3–xTe4 and La3–xTe4, showing a wider range in Nd3–xTe4.(13) The increase in mobility agrees with the decrease in carriers. SPB modeling was based on the Nd2.78Te4 sample with nH = 2.72 × 1021 cm–3 with T = 600 K, m* = 3.93me, μ0 = 4.45 cm2/V·s, and κL = 0.56 W/m·K. (D) High-temperature electron mobility for Nd3–xTe4 compared with La3–xTe4.(13) The change in behavior near 850 K indicates the activation of a higher energy band from broadening of the Fermi–Dirac distribution.

Figure 5. (A) Seebeck vs temperature for Nd3–xTe4 compared against data for La3–xTe4.(13) Nd3–xTe4 showed a near 20% increase in Seebeck at similar vacancy concentrations. (B) Pisarenko plot (Seebeck vs carrier concentration) at 300 K comparing single-band (SPB) models for Nd3–xTe4 with different effective masses and a semi-empirical multiband model for La3–xTe4 at 300 K showing similar transport at room temperature.(13) (C) Pisarenko plot at 600 K comparing single-band (SPB) models for Nd3–xTe4 with different effective masses and a semi-empirical multiband model for La3–xTe4 at 600 K showing a departure of Nd3–xTe4 from the model for La3–xTe4, requiring higher masses to describe the transport in Nd3–xTe4.(13) (D) Power factor (S2/ρ ) vs temperature for various vacancy concentrations.(13) The similar power factor of Nd3–xTe4 compared to La3–xTe4 results from the gains in the Seebeck coefficient of Nd3–xTe4 being offset by the increased resistivity.

Figure 6. (A) Temperature-dependent thermal conductivity of Nd3–xTe4 compared to that of La2.74Te4.(13) (B) Lattice thermal conductivities of Nd3–xTe4 calculated using the Wiedemann–Franz law, compared to that of La2.74Te4.(13) The similarity in κL of Nd3–xTe4 and La3–xTe4 indicates that differences in total thermal conductivity are from differences in the electronic contribution to κ. Higher carrier concentration Nd3–xTe4 samples were omitted because of strong deviations from the Wiedemann–Franz law. (C) zT vs temperature for Nd3–xTe4 compared to that of La2.74Te4.(13) A zT of 1.2 was achieved using Nd2.78Te4 at 1273 K, representing a 10% improvement over La2.74Te4. (D) zT as a function of carrier concentration at 600 K for Nd3–xTe4.
Concluding comments from the paper:
We employed DFT to perform band structure calculations for Nd3Te4 and La3Te4 to compare the impact of the four 4f electrons of Nd to the transport properties of Nd3–xTe4, which predicted a sharp peak in the DOS situated near the Fermi level in Nd3Te4. A higher Seebeck coefficient was predicted for Nd3Te4 when compared to La3Te4, in agreement with the predicted increase in the DOS near the Fermi level. Nd3–xTe4 was synthesized over a range of vacancy concentrations and was found to exhibit the metastable cubic Th3P4 structure type for lower vacancy concentrations after mechanochemical synthesis. Phase purity of the Th3P4 structure was confirmed via X-ray diffraction, and sample compositions were analyzed using wavelength-dispersive spectroscopy. The electronic and thermal properties were measured, and Nd3–xTe4 was found to have a higher resistivity and higher Seebeck coefficient at equivalent vacancy concentrations, as well as lower electronic contribution to thermal conductivity than La3–xTe4. With regard to thermoelectric performance, the increase in Seebeck coefficient was offset by a corresponding increase in resistivity.(28) However, this increased resistivity also served to lower the thermal conductivity. A zT = 1.2 was achieved at 1273 K for Nd2.78Te4, which is a 10% improvement over that of La2.74Te4. If used in future RTGs, Nd3–xTe4 will serve to increase the thermal-to-electrical conversion efficiencies in these devices, enabling higher specific power densities and reduced dependence on precious radioisotope fuel.
Right now, the Pu-238 radioisotope fuel
is precious, although it occurs to me that there might be a better way to make it than the traditional approaches, involving fluid phase fuels.
I personally have convinced myself that the
only way to clean up the increasingly destroyed atmosphere (and many other intractable environmental problems) is via very high temperature nuclear reactors, reactors operating at temperatures high enough to split either water into hydrogen and oxygen (via thermochemical cycles) or carbon dioxide into carbon monoxide and oxygen. Many such cycles are known.
There are many approaches to recovering energy and raising efficiency of high temperature devices, including combined cycle approaches using Brayton/Rankine/Stirling cycles in tandem,
or perhaps, thermoelectric devices such as are described here.
A note on tellurium: I'm quite sure that somewhere, maybe here, I have ridiculed cadmium telluride solar cells, noting that tellurium, besides its toxicity, is a critical element that is not really "renewable" but easily subject to depletion.
Tellurium is a fission product, and in fact, it has some materials science problems associated with it. Here for instance is one example, described in a recent publication of a class of nuclear reactors known as "molten salt reactors" (MSRs):
3.4.1 Tellurium embrittlement
Chalcogens all exhibit a high electron affinity, being members of Group 16, and Te is third highest in the column at 190kJ-mol~^ (1.96 eV). The ground-state electronic configurationsfor oxygen and tellurium (O = Is, 2s2 2p4; Te = [Kr] 4d10 5s2 5p4) both exhibit incomplete p orbitals (np4); a filled p orbital has six electrons. These incomplete p orbitals make chalcogens aggressive oxidizing agents, so as to close that deficit, namely, achieve a closed np* (O, n = 2; Te, n = 5) orbital. For technical reasons beyond the scope of this discussion, sulfur, selenium, and tellurium can adopt higher oxidation states. In the case of tellurium in the MSR experiments conducted at ORNL, Te was found as TeF6, a highly soluble form of a tellurium fluoride with a formal charge of + 6. Early MSRE studies demonstrated the damage that Te inflicted on the inner surfaces of containment materials. In some cases fissures up to 300 pm deep were produced (Houtzeel and Dyer, 1972; McCoy, 1978). In a series of investigations, ORNL researchers examined the effects of TeF6 and TeF6 + F2 exposure to a number of alloys, the most promising of which for use in an MSR (at the time) were Nickel 200 (99 + % pure Ni) and Monel K-500 (67%Ni, 32.x% Cu, balance other metals); Hastelloy was not included in these experiments. Both alloys were tested at approximately 500°C and for varying lengths of time. Both were found to have substantial Te-derived corrosion, both at the surface and intergranularly, although the Monel K-500 less so. One possibility is that, despite the presence of highly oxidizable Ti and Al in the Monel K-500 in a fluorinating environment such as in the molten-salt bath, the intermetallic, gamma prime precipitate (NiaAl/Ti, FCC crystal structure) may assist in corrosion resistance. When these alloys were exposed to TeFg and F2, corrosion rates were reduced. It is possible that this reduction is attributable to F2 being such a strong oxidizer, thereby maintaining TeFg in a fully oxidized, + 6 state.TeFg, the solubilized Te species, was readily reduced when exposed to these and other alloys. In fact, some metallic Te was detected on the Monel coupons, as well as CuTe. When investigated under x-ray crystallography, NiTc2, NiTe and NiF2 were all found in varying amounts on each coupon, supporting typical chalcogenic behavior where a Ch prefers a formal charge of - 2. Cu2-xTe was also found on the Monel K-500, as would potentially have been predicted, given the percent balance that Cu makes as the Monel alloy. Cu2-x may also have been a logical prediction as a mixed system, given Cu’s two oxidation states. This was, in part, because Te, like O, Se, S, all prefer a Ch-2 (Ch = chalcogen) state, to close that p orbital…
...and so on. At ONRL the oxidation in the MSR was controlled by placing reducing agents in the fluid fuel, specifically uranium trifluoride.
(Cf. Thomas Dolan, Ed. Molten Salt Reactors and Thorium Energy, Woodhead Publishing, 2017 pg. 56)
For the record, not that anyone is likely to care, although I was once quite fond of MSR's and still believe that molten salt technologies have potential uses, more in fuel reprocessing than in reactors although
some MSR type reactors are better than others, I have changed my mind largely about them and am considering what I regard as better options.
Lanthanum and neodymium in any case are also fission products, and can be recovered from used nuclear fuels as tellurium can be.
The difference here is that solar cells have low capacity utilization - they only operate near their peaks (but seldom, often
never, at their quoted peaks) when the sun is up on low cloud days - whereas a thermoelectric device, as is the case on the Voyager and New Horizon missions can operate continuously. Moreover, they can be far more dense in material usage than solar cells, and the risk of toxicity is lower simply because the tellurium is not
distributed to the masses, many of whom lack the education to understand the risks of tellurium (and cadmium) or proper routes to disposal.
I note that if the pools containing the spent fuel rods at Fukushima had been empty because the used fuel was contained in thermoelectric devices rather than in water, the reactors would still be operating, even after being swamped with seawater, since the circulating pumps would not have depended on diesel back up generators, but simply on a thermal gradient is a solid state device.
Just saying...
I trust you're having a wonderful evening.