Genome editing retraces the evolution of toxin resistance in the monarch butterfly.
The paper I'll discuss in this post is this one: Genome editing retraces the evolution of toxin resistance in the monarch butterfly. (Whiteman et al, Nature 574, 409–412 (2019))
One of the happiest memories of the childhood of my sons was when we went to a local park, the New Jersey side of Washington's Crossing Park, where the ranger showed us how to collect monarch butterfly eggs, which were laid on the underside of milkweed plants, poisonous plants that grow wild all around here. We took the leaves home, put them in a butterfly cage, and ultimately the eggs hatched, and caterpillars began munching the leaves. We keep collecting them from the fields around here, until they formed cocoons, pupae, and finally emerged as butterflies, which we released.
It was a beautiful, wonderful experience, and probably one I would have never had were I not a father.
The Monarch's don't really "migrate" as individuals; each year several generations make their way across North America from Mexico, breeding repeatedly, happily munching toxic milkweed all across America.
A truly wondrous life form!
CRISPR/CAS-9 is a gene editing tool developed by Jennifer Doudna and Emmanuelle Charpentier that utilizes a bacterial protein, known as CAS-9, which is in a sense an "immunity defense" for procaryotic organisms, that operates in conjunction with a guide RNA sequence that acts much like "interfering RNA," "iRNA" relying on complementary. By appropriate editing of the RNA sequence, this system can be modified for the purpose of gene editing, both for research purposes and, perhaps, for therapeutic modalities.
(Having participated in my career in a number of "really hot" biomedical fads, I tend to be more "wait and see" than over the top enthusiastic for these sweeping claims.)
The paper cited above is using CRISPR/Cas-9 as a research tool to discover how the Monarch butterfly became immune to the plant toxins in milkweed. This toxicity by the way, protects the Monarch from predators, since the butterflies themselves are toxic.
From the abstract:
Letter
Published: 02 October 2019
Genome editing retraces the evolution of toxin resistance in the monarch butterfly
Marianthi Karageorgi, Simon C. Groen, Fidan Sumbul, Julianne N. Pelaez, Kirsten I. Verster, Jessica M. Aguilar, Amy P. Hastings, Susan L. Bernstein, Teruyuki Matsunaga, Michael Astourian, Geno Guerra, Felix Rico, Susanne Dobler, Anurag A. Agrawal & Noah K. Whiteman
Nature volume 574, pages409–412 (2019) | Download Citation
Abstract
Identifying the genetic mechanisms of adaptation requires the elucidation of links between the evolution of DNA sequence, phenotype, and fitness1. Convergent evolution can be used as a guide to identify candidate mutations that underlie adaptive traits2,3,4, and new genome editing technology is facilitating functional validation of these mutations in whole organisms1,5. We combined these approaches to study a classic case of convergence in insects from six orders, including the monarch butterfly (Danaus plexippus), that have independently evolved to colonize plants that produce cardiac glycoside toxins6,7,8,9,10,11. Many of these insects evolved parallel amino acid substitutions in the α-subunit (ATPα ) of the sodium pump (Na+/K+-ATPase)7,8,9,10,11, the physiological target of cardiac glycosides12. Here we describe mutational paths involving three repeatedly changing amino acid sites (111, 119 and 122) in ATPα that are associated with cardiac glycoside specialization13,14. We then performed CRISPR–Cas9 base editing on the native Atpα gene in Drosophila melanogaster flies and retraced the mutational path taken across the monarch lineage11,15. We show in vivo, in vitro and in silico that the path conferred resistance and target-site insensitivity to cardiac glycosides16, culminating in triple mutant ‘monarch flies’ that were as insensitive to cardiac glycosides as monarch butterflies. ‘Monarch flies’ retained small amounts of cardiac glycosides through metamorphosis, a trait that has been optimized in monarch butterflies to deter predators17,18,19. The order in which the substitutions evolved was explained by amelioration of antagonistic pleiotropy through epistasis13,14,20,21,22. Our study illuminates how the monarch butterfly evolved resistance to a class of plant toxins, eventually becoming unpalatable, and changing the nature of species interactions within ecological communities2,6,7,8,9,10,11,15,17,18,19.
An excerpt of the introductory text:
Convergently evolved substitutions in ATPα have been hypothesized to contribute to cardiac glycoside resistance in the monarch butterfly and other specialized insects via target-site insensitivity (TSI) in the sodium pump6,7,8,9,10,11. However, it is unclear whether the changes are sufficient for resistance in whole organisms6,7,8,9,10,11,15,18,23 or are ‘molecular spandrels’—candidate adaptive alleles that do not confer a fitness advantage when tested more rigorously1,5. In addition, the evolutionary order of substitutions suggests a constrained adaptive walk11,13,14,20,21,22,24, but an in vivo genetic dissection has not been conducted, so it is not possible to draw conclusions about the adaptive role of these substitutions1,2,3,4,5,15.
We have identified a core set of amino acid substitutions in cardiac glycoside-specialized insects that define potential mutational paths to resistance and TSI. We focused on the first extracellular loop (H1–H2) of ATPα, where most candidate TSI-conferring substitutions occur7,8,9,10,11 (Fig. 1a). We used maximum likelihood to reconstruct ancestral states for cardiac glycoside specialization (feeding and sequestering) and amino acids within the H1–H2 loop of ATPα across a species phylogeny...
The authors identified a series of known amino acid substitutions in the ATPα in the Monarch, at residues 111, 119, and 122.
A figure from the text:
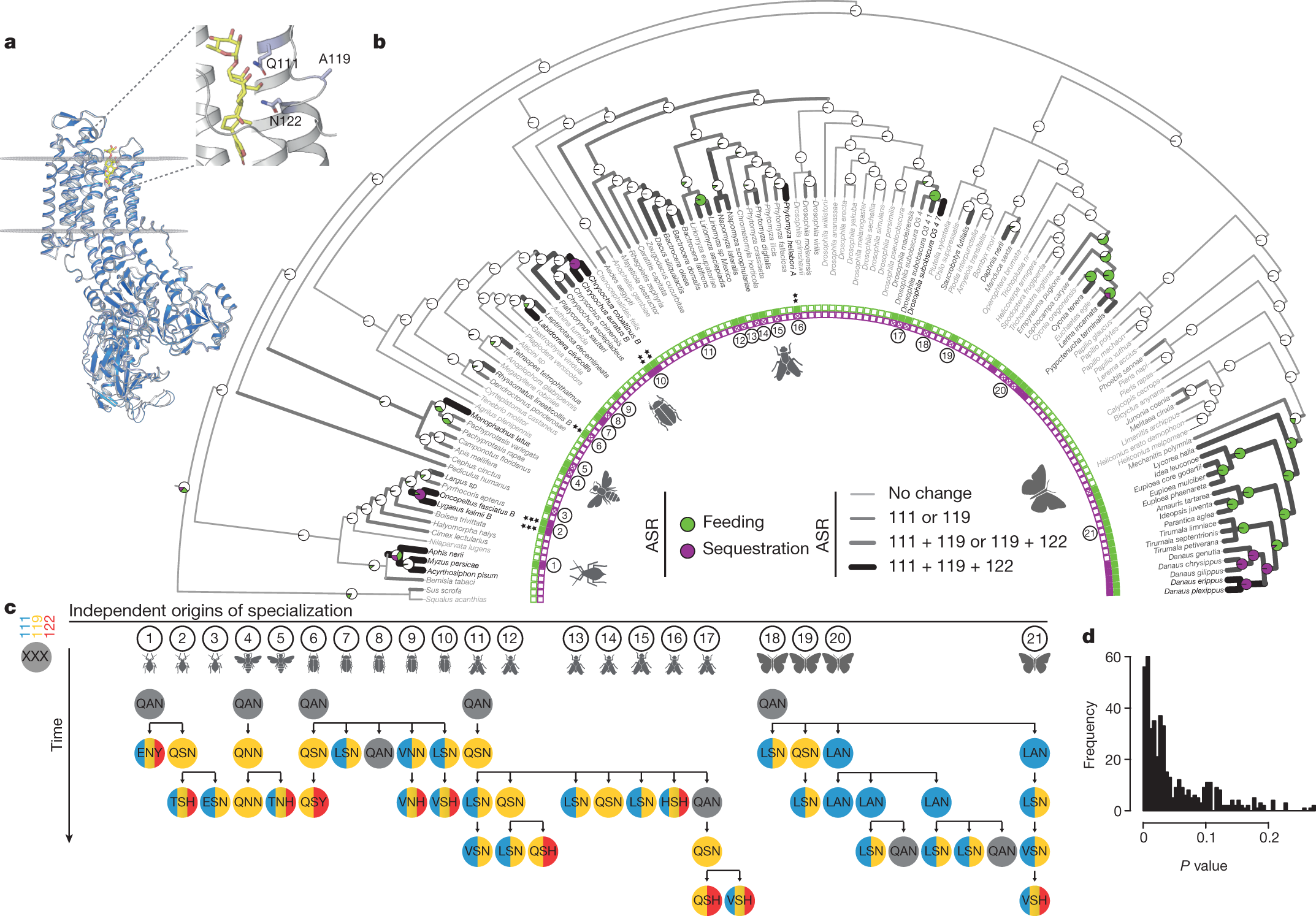
The caption:
a, Protein homology model of Drosophila melanogaster ATPα (navy) superimposed on a Sus scrofa ATPα crystal structure (light grey) with ouabain (yellow) in the binding pocket. Residues 111, 119 and 122 (sticks) within the H1–H2 extracellular loop are associated with feeding on cardiac glycoside-producing plants and toxin sequestration. b, Maximum likelihood phylogeny based on 4,890 bp from Atpα and coi, with maximum likelihood ancestral state reconstruction (ASR) of feeding and sequestering states, estimated from the states of extant species (inner band of squares). Reconstructions are shown as nodal pie graphs (white, neither feeding nor sequestering; green, feeding; purple, feeding and sequestering), and the number of substituted sites at positions 111, 119 and 122 along branches in grey-scale (light grey 0, medium grey 1, dark grey 2, black 3), based on maximum likelihood ASR of H1–H2 loop amino acid sequences. Black asterisks indicate the Atpα copy number for species with multiple paralogues. c, ATPα substitutions inferred from ASR at positions 111 (blue), 119 (yellow) and 122 (red) in 21 lineages where specialization occurred independently. d, P value distribution from a set of randomized tests to determine the reproducibility of substitutions observed along mutational paths among sub-sampled groups compared to randomly permuted substitutions. On average, 4.9% (considering all mutational steps) of randomly permuted trajectories demonstrate a degree of ordering equal to or greater than observed mutational paths.
The DNA of the
Drosophilia fly was modified via editing to provide the necessary homology allowing the organisms to feed on cardiac glycosides as well.
Another figure from the paper:
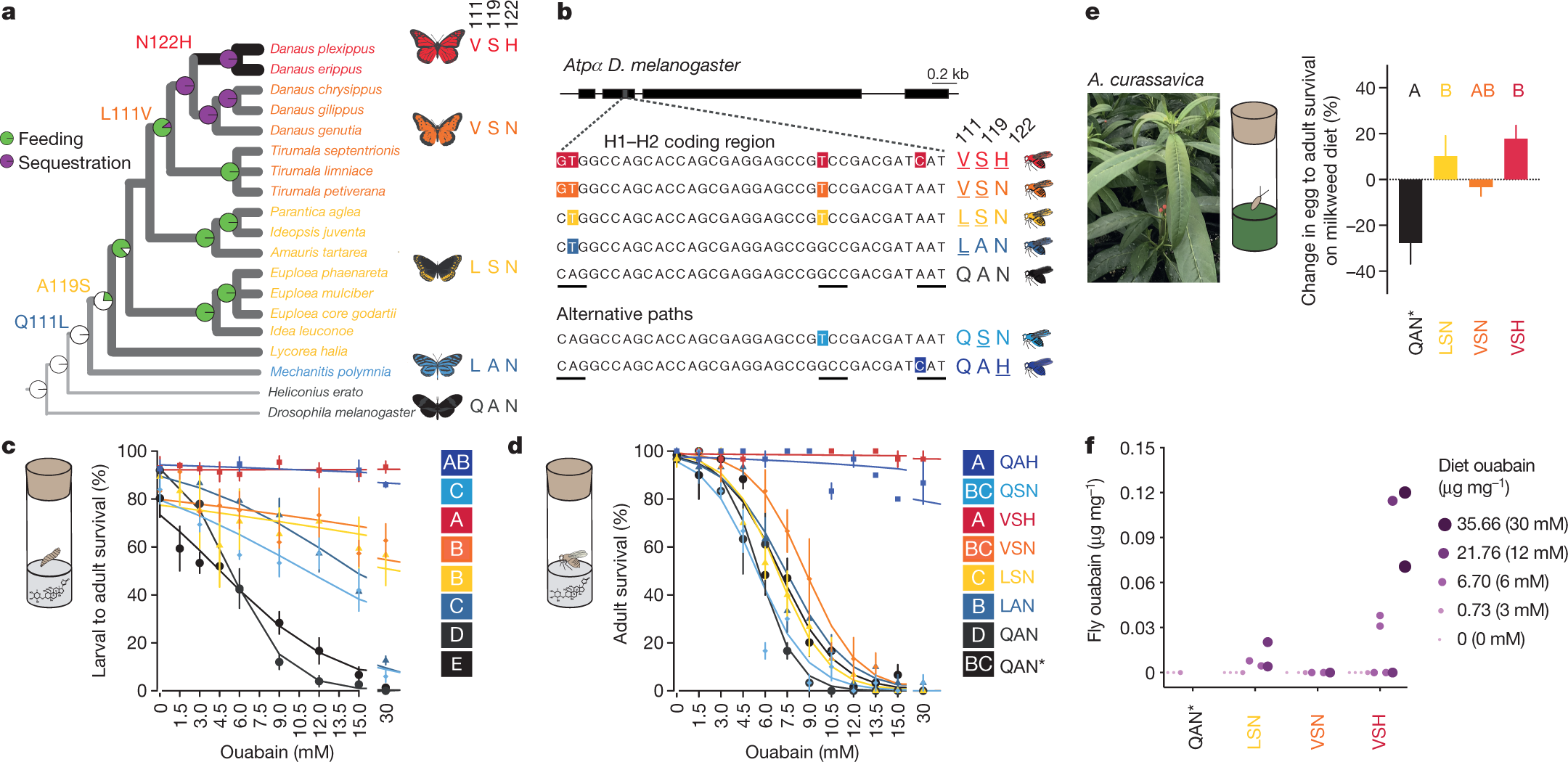
a, The monarch butterfly lineage with the substitutions observed in the H1–H2 loop of ATPα (adapted from Petschenka et al.)11. b, Non-synonymous point mutations in the edited DNA sequence of the native Atpα in Drosophila knock-in lines code for the substitutions at sites 111, 119 and 122. Codons are underlined. c, d, Larval–adult survival (c) and adult survival (d) of flies reared on diets with ouabain were different between monarch lineage knock-in lines and control lines (QAN = engineered control; QAN* = w1118 wild type). Symbols represent the mean ± s.e.m. of 3–6 biological replicates (50 larvae and 10 females per replicate in c and d, respectively). Curves were fit using a logistic regression model for each line. Pairwise differences in survivorship trajectories between lines were evaluated with a likelihood ratio test on the significance of the interaction term between genotype (line) and ouabain concentration in a logistic regression for each pair of lines (letters). e, Egg–adult survival on diet supplemented with Asclepias curassavica leaves relative to control diets (n = 3–4; 100–200 eggs per replicate, see Methods; mean ± s.e.m.) was different between monarch lineage knock-in lines and QAN* (one-way ANOVA, P = 0.0035 followed by post hoc Tukey’s tests (letters)). f, Ouabain concentrations in diet versus adult fly bodies among monarch lineage knock-in lines (n = 2–4 biological replicates per group). Adult flies had not fed since eclosion. Genotype and dietary ouabain concentration influenced the probability of detecting ouabain in post-eclosion flies (logistic regression and likelihood ratio test, genotype two-sided P = 0.024, dietary ouabain concentration two-sided P = 6.344 × 10−5). Further information on experimental design and statistical test results is found in the Source Data.
Some observations:
We obtained in vivo evidence for adaptation in monarch lineage Atpα through larval–adult and adult survival experiments. Knock-in fly lines were reared on yeast medium with increasing concentrations of ouabain, a hydrophilic cardiac glycoside6 (Fig. 2c, d, Extended Data Figs. 4, 5). LAN, the first genotype to evolve, increased larval–adult survival at lower ouabain concentrations, but survival declined sharply as concentrations increased. LAN also increased adult survival at lower ouabain concentrations. LSN, the second genotype to evolve, increased larval–adult survival at the highest ouabain concentrations. The next step, VSN, provided the same larval–adult and adult survival benefit as LSN. Finally, the survival of ‘monarch flies’ carrying the monarch butterfly genotype (VSH) was unaffected by even the highest levels of ouabain in larvae and adults6,9,11,18 (Fig. 2c, d), which was not due to reductions in feeding rate or toxin ingestion (Extended Data Fig. 6).
When knock-in line eggs were placed on medium containing the suite of cardiac glycosides found in the leaves of the milkweed species Asclepias curassavica and A. fascicularis6, monarch lineage fly genotypes generally showed increased egg–pupal and egg–adult survival rates (Fig. 2e, Extended Data Fig. 7), although not always for VSN (Extended Data Figs. 3, 7). The LSN, VSN and VSH genotypes may enable insects to cope with the complex milieu of cardiac glycosides encountered during host shifts to these plants.
The monarch butterfly ATPα substitutions at positions 111, 119 and 122 may unlock a passive evolutionary route to cardiac glycoside sequestration, as we found small amounts of ouabain in newly emerged adult ‘monarch flies’ reared as larvae on a diet containing ouabain (Fig. 2f). However, toxin concentrations were far lower than in monarch butterflies, and the location of ouabain in flies is unclear6,17,18.
Another graphic:
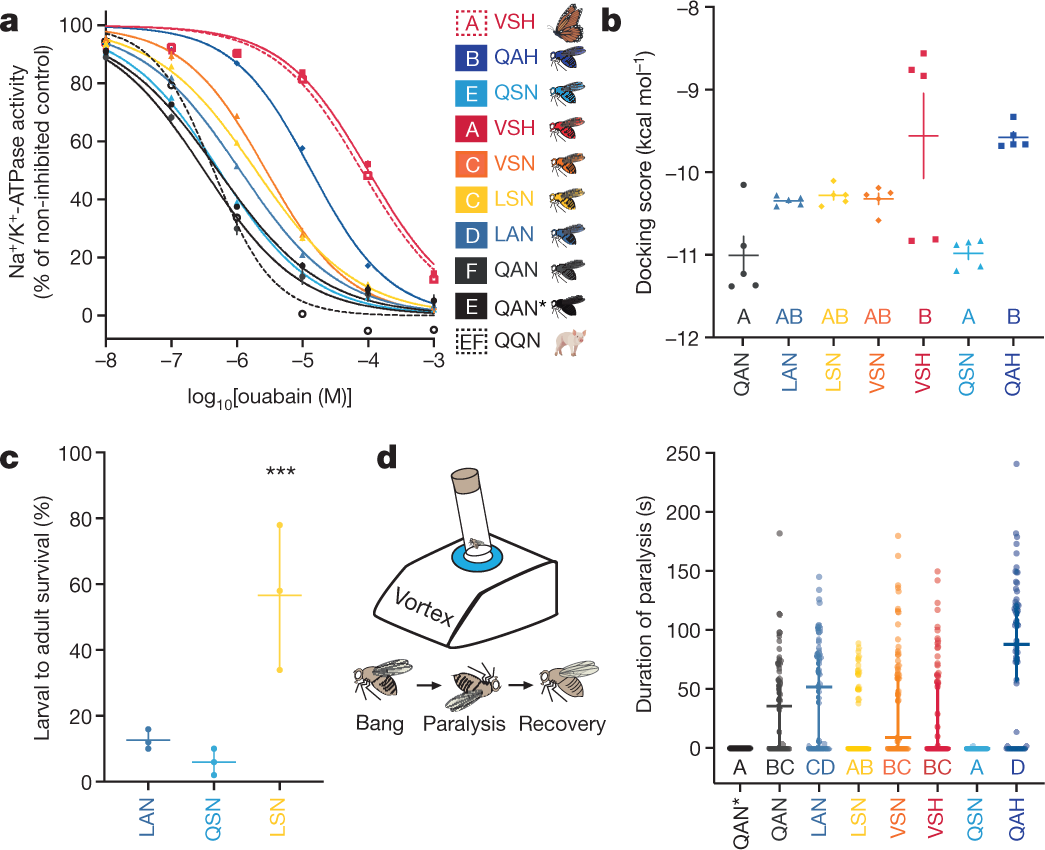
The caption:
a, In vitro ouabain sensitivity of Na+/K+-ATPase activity in extracts of monarch lineage knock-in and control line fly heads (solid lines; QAN, engineered control; QAN*, w1118 wild type), against activity in extracts of monarch butterfly and pig nervous tissue (positive and negative control, dashed red and black line, respectively). Symbols represent the mean ± s.e.m. of 3–7 biological replicates. log10[IC50] (half-maximum inhibitory concentration) values for the Na+/K+-ATPases were estimated after fitting four-parameter logistic regression curves, and were different between genotypes (one-way ANOVA (P < 0.0001) with post hoc Tukey’s tests (letters)). b, Mean docking scores (± s.e.m. of five replicate calculations) from molecular simulations of ouabain binding to the Na+/K+-ATPases found along the monarch lineage showed differences between genotypes (one-way ANOVA (P = 0.0001) with post hoc Tukey’s tests (letters)). c, Effects of the substitutions Q111L, A119S and their combination on larval–adult survival on diets with 30 mM ouabain. Symbols represent the mean ± s.e.m. of three biological replicates (50 larvae each). The effect of mutations A119S and Q111L together was nearly threefold greater than the combined individual effects on survivorship (logistic regression, interaction effect between mutations: ***P = 2.36 × 10−15), indicating positive epistasis. d, Duration of paralysis following mechanical shocks (that is, bang sensitivity; n = 60 five-to-six-day-old adult flies). Bang sensitivity was affected by genotype (Kruskal–Wallis test (P < 0.0001) with post hoc Dunn’s multiple comparisons tests (letters); medians with 95% confidence intervals), and was higher for QAH than for all other genotypes (P < 0.05), except for LAN, which showed higher bang sensitivity than LSN (P = 0.0134). Further information on experimental design and statistical test results can be found in the Source Data.
The full paper makes comments about the evolutionary pathway by which the TSI, Target substance insensitivity, to cardiac glycosides evolved.
(The old drug digitalis and related digoxin fit into this class of compounds.)
Some concluding remarks:
Substitutions at three amino acid sites in ATPα are sufficient together, but not alone, to explain the evolution of resistance and TSI to cardiac glycosides achieved by the monarch butterfly at organismal, physiological and biochemical levels. The adaptive walk follows theoretical predictions on the length of such walks2,3,4,13,14, involves epistasis13,14,20,22, and minimizes pleiotropic fitness costs3,4,13,14,21, and variations of it convergently re-appeared across lineages that diverged more than three hundred million years ago7,8,9,10,11. Genome editing technology facilitates functional tests of adaptation across levels of biological organization5,25,26. Although mutational paths to adaptive peaks have been identified in microorganisms2,3,4,13,14,22, this is, to our knowledge, the first in vivo validation of a multi-step adaptive walk in a multicellular organism, and illustrates how complex organismal traits can evolve by following simple rules.
This technology is very powerful.
Like all powerful technologies, it has a potential to do good and great things, and likewise, bad and terrible things. The choice is moral. As old and as cynical as I am, I still believe, in spite of it all, in the capacity for humanity to come down on the side of good and great.
Have a nice day tomorrow.



