Desymmetrization of difluoromethylene groups by C-F bond activation
Last edited Sat Jul 25, 2020, 11:33 AM - Edit history (1)
The paper I'll discuss in this paper is this one: Desymmetrization of difluoromethylene groups by C–F bond activation (Hartwig et al., Nature volume 583,548–553 (2020))
Whether it's known in the general public or not, the carbon fluorine bond, one of the most stable bonds known, is one of the most troubling issues in the environment; a subject about which I've written in this space previously.
Although the use of PFOS, PFOA and related compounds have been largely banned around the world, except in countries run by right wing morons who hate science and the environment, the situation is far from being resolved. There are, for example, technological efforts, such as the ill advised and environmentally dubious efforts to store energy to correct to address the issue that makes so called "renewable energy" environmentally, economically, and thermodynamically unacceptable and unsustainable. The much discussed approach to energy storage, and one that has been commercialized is the fuel cell, many of which have fluoropolymeric solid electrolytes. Some people are so thoughtless and rote that they thing hydrogen is a "clean fuel." It isn't. It's a thermodynamic and environmental nightmare, at least as it is currently made, and might be made in the quixotic adventure to make so called "renewable energy" matter.
Anyway.
I have thought a great deal about fluorine carbon bonds in my long life; particularly as I dig deeper into environmental issues, but this paper is more relevant to my initial interest in them, since they are, owing to their strong electron withdrawing nature coupled with an atomic radius close to that of hydrogen, very important in medicinal chemistry. Here's a nice review on the topic:
Applications of Fluorine in Medicinal Chemistry (Gillis et al., J. Med. Chem. 2015, 58, 21, 8315–8359)
Of course, in the "every problem is a nail if you only have a hammer" fashion, my feel for the means to address the flourine carbon bond problem in the environment is high energy radiation, given my fondness for the wonderful properties of used nuclear fuels - the materials that people with poor imaginations have named so called "nuclear waste" but anything that involves the activation of carbon flourine bonds captures my eye.
This one involves making chiral molecules from difluoromethylenes. It's pretty cool.
From the text:
One of the most common approaches to the enantioselective synthesis of chiral molecules is the selective replacement of one of the two enantiotopic C–H bonds of a methylene unit to form a tertiary stereogenic centre. This approach is exemplified by classic α-functionalizations of carbonyl compounds7,8, asymmetric lithiations9, as well as modern catalytic reactions, such as benzylic functionalizations10,11, carbene or nitrene insertions12,13 and directed C–H activations14 (Fig. 1a). Although the synthesis of organic compounds with fluorine atoms installed in specific positions is often essential to control the physiochemical properties, such as the pKa, lipophilicity, conformation and metabolic stability of pharmaceutical candidates2, the analogous desymmetrizations of difluoromethylene units to form enantioenriched tertiary alkyl fluorides, which are bioisosteres for common tertiary stereocentres3 (Fig. 1b), have not been reported so far. This class of reaction would provide a particularly valuable route to tertiary alkyl fluorides because the starting difluoromethylene groups are readily installed by classical deoxyfluorination reactions or modern difluoroalkylations15,16,17,18,19.
Figure 1:
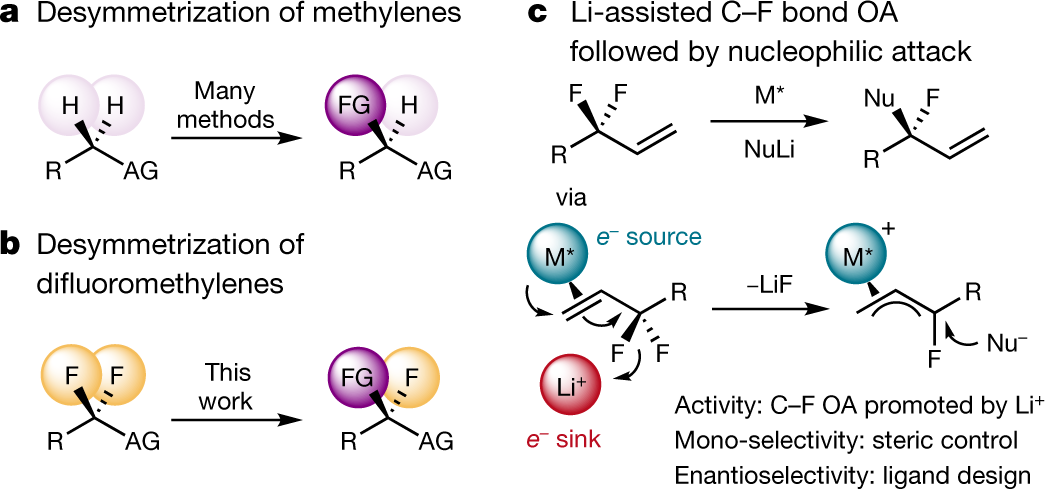
The caption:
a, The desymmetrization of methylene groups by enantioselective C–H activation is a general strategy for the construction of simple tertiary stereocentres. b, The analogous desymmetrization of difluoromethylene groups by enantioselective C–F activation has not been reported so far. c, The cooperation of a chiral, low-valent transition metal complex and a hard fluorophilic activator enables the enantioselective activation of a single C–F bond at an allylic position. FG, functional group; AG, activating group (for example, electron-withdrawing group, aryl group, heteroatom or directing group; few examples lack an activating group); Nu, nucleophile; M, transition metal; OA, oxidative addition.
The authors discuss their thinking further and then discuss their approach to this novel chemistry:
Herein, we report the catalytic, enantioselective replacement of one of two fluorine atoms in geminal difluorides to form enantioenriched tertiary allylic fluorides in high yield, high regioselectivity, high enantioselectivity and perfect chemoselectivity for the activation of a single C–F bond. The reaction occurs by combining a tailored iridium phosphoramidite catalyst with an appropriate counterion or silyl group on the nucleophile to labilize the fluoride and to deliver the nucleophile to the fluorine-containing site on the allyl electrophile. This ‘push–pull’ cooperation between a soft transition metal and a fluorophilic activator accelerates the oxidative addition of C–F bonds and is shown to extend to the activation of C–F bonds at less reactive benzylic positions.
Iridium is, one should note, a very expensive and rare metal; its cogener, rhodium is also important in asymmetric chemistry, supplies of rhodium can be maintained by isolation from used nuclear fuel although it is fairly rare (and thus expensive) when obtained by mining.
Phosphoamidites are well known, and very important in synthetic nucleic acid chemistry; and are in general no big deal.
Their catalyst structure is shown in this figure:
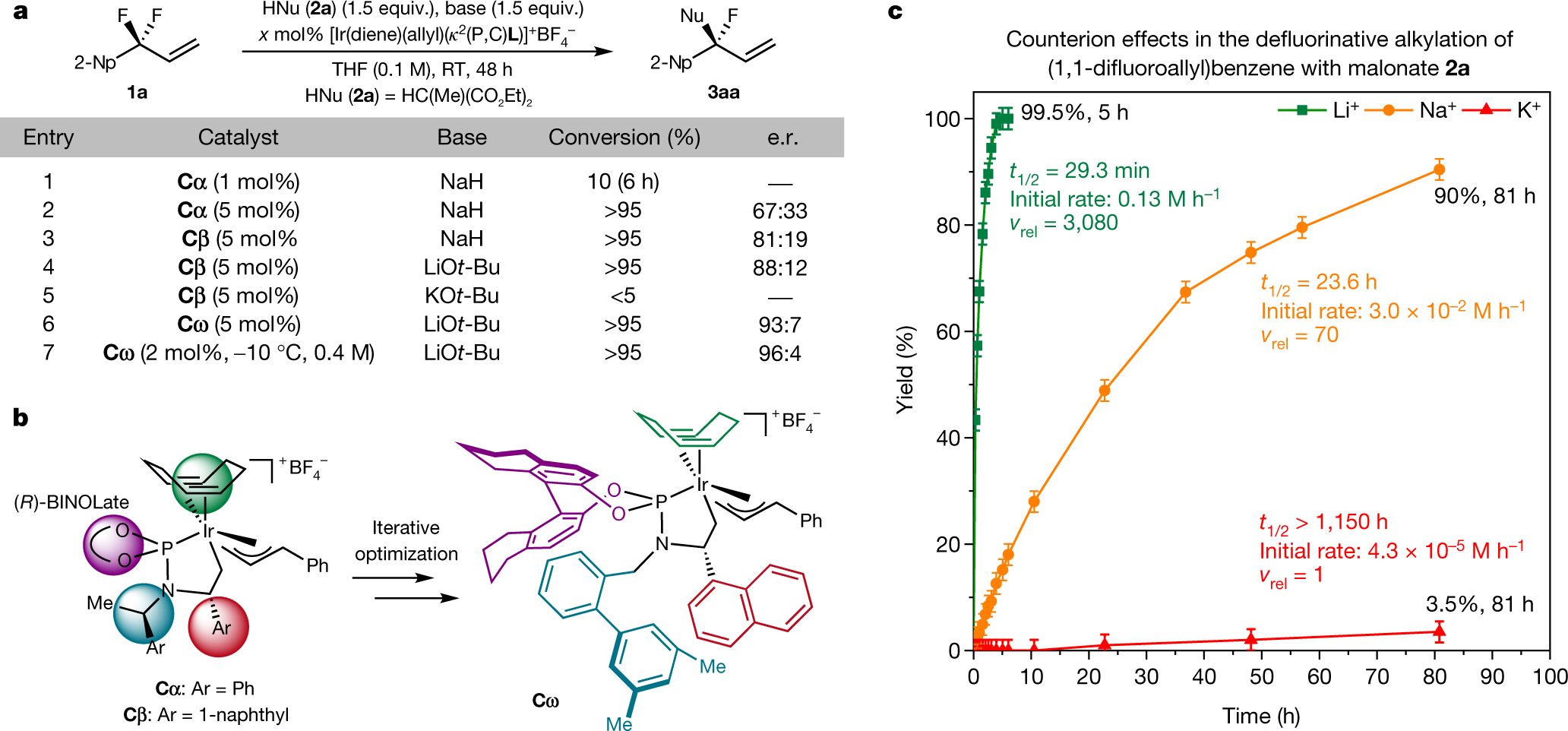
The caption:
The identity of the fluorophilic cation strongly affects the rate of C–F activation. Modulation of the steric profile of the cyclometalated iridium catalyst improves the enantioselectivity. a, Effect of the catalyst, the catalyst loading and the base on conversion and enantioselectivity. The conversion of the starting material is determined by 1H NMR spectroscopy. The enantiomeric ratio (e.r.) is determined by chiral high-performance liquid chromatography (HPLC) after purification by preparative thin-layer chromatography (TLC). b, Development of a highly enantioselective iridium catalyst. c, Quantification of the role of the cation in C–F activation reactions. Kinetic studies were conducted according to the scheme in a with substrate 1b (PhCF2CH = CH2) and catalyst Cω (4 mol%). Error bars in c correspond to ±2% yield error associated with quantitative NMR spectroscopy. THF, tetrahydrofuran; 2-Np, 2-naphthyl; Ph, phenyl; Ar, aryl; Me, methyl; BINOLate, 1,1′-bi(2-naphtholate); RT, room temperature; vrel, relative rate; κ denotes binding of non-contiguous atoms in a chelating ligand.
It is interesting to note, since this caption mentions chiral HPLC, that fluoropolymers - not that I endorse fluoropolymers in the world as it is - could, conceivably make very hydrophobic chiral stationary phases.
The authors explore the broad synthetic utility of their discovery:
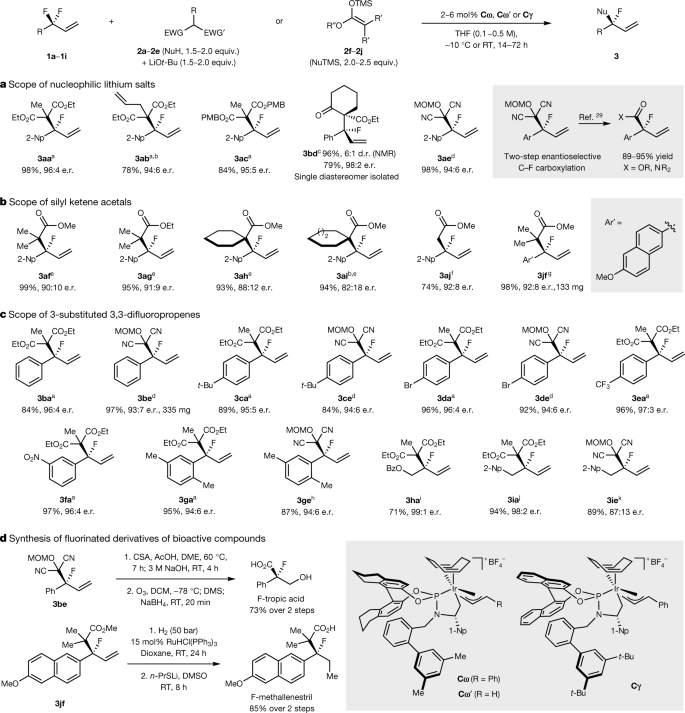
The caption:
a, Nucleophilic lithium salts in defluorinative alkylation reactions. b, Silyl ketene acetals in defluorinative alkylation reactions. c, 3-Substituted 3,3-difluoropropenes in defluorinative alkylation reactions. d, Synthesis of fluorinated derivatives of medicinally active compounds from the substitution products. Isolated yields reported unless otherwise noted. a2 mol% Cω, –10 °C, 72 h. b96 h. c5 mol% Cω', 0 °C, 72 h. d2 mol% Cω', 5 equiv. LiBr, RT, 24 h. e6 mol% Cγ, 5 mol% NaCMe(CO2Et)2, RT, 48 h. ftert-Butyl dimethyl silyl ketene acetal, 6 mol% Cγ, RT, 53 h. g2 mol% TMSOTf, 4 mol% Cγ, dioxane, RT, 40 h. h5 mol% Cω', 5 equiv. LiBr, RT, 48 h. i5 mol% Cω, 65 °C, 96 h. j2 mol% Cω, 3 equiv. Ba(OTf)2, RT, 19 h. k20 mol% Cω, 3 equiv. Ba(OTf)2, dioxane, RT, 24 h. EWG, electron-withdrawing group; TMS, trimethylsilyl; MOM, methoxymethyl; Bz, benzoyl; CSA, camphor sulfonic acid; DME, 1,2-dimethoxyethane; DMS, dimethylsulfide; 1-Np, 1-naphthyl; d.r., diastereometric ratio.
The authors extended their work to palladium catalysts:
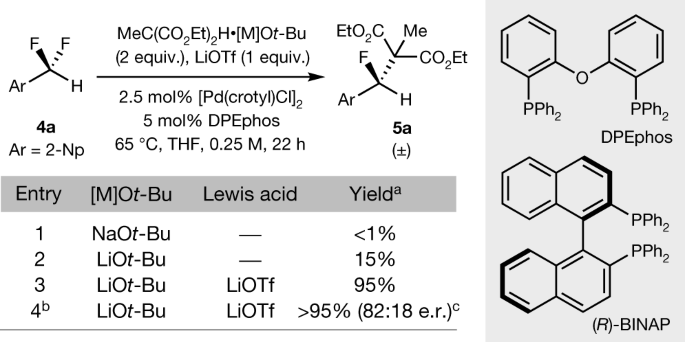
The caption:
The cooperation between a low-valent transition metal and a fluorophilic cation enables the activation of benzylic C–F bonds in difluoromethylarenes, and reactions conducted with a chiral ligand are enantioselective. aYield determined by 1H and 19F NMR spectroscopy. bReaction conducted with 5 mol% [Pd(crotyl)Cl]2 and 15 mol% (R)-BINAP. cEnantiomeric ratio determined by chiral HPLC. LiOTf, lithium triflate.
Palladium is also a potentially valuable element available from used nuclear fuel, although to minimize its radioactivity - not that radioactive palladium is as much of a problem as people might think, and probably
do think in the age of stupidity - it should be isolated very quickly from ruthenium-106 before it is allowed to decay, owing to the long half life of Pd-107.
An exploration of the reaction mechanism.
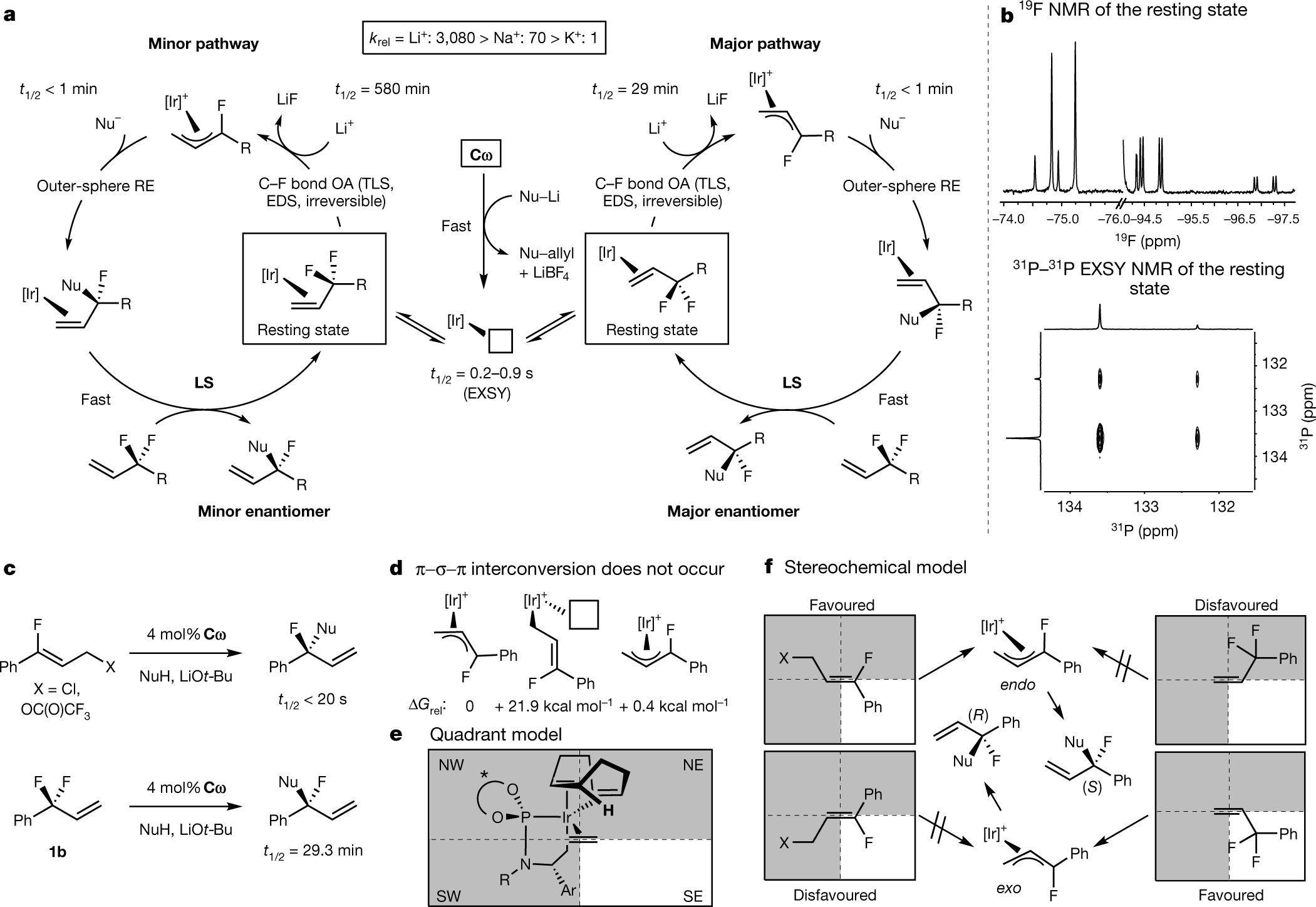
a, Proposed mechanism. b, NMR spectroscopic characterization of the catalyst resting state. c, Comparison of allylic fluoroalkylation reactions with 3-fluorocinnamyl electrophiles and 3-substituted 3,3-difluoropropenes. d, η1 allyl intermediates are too high in energy to participate. e, Quadrant diagram for Ir(I) olefin complexes. f, Stereochemical model for 3-fluorocinnamyl electrophiles and 3-substituted 3,3-difluoropropenes; see Supplementary Information for a more detailed discussion. OA, oxidative addition; RE, reductive elimination; LS, ligand substitution; TLS, turnover-limiting step; EDS, enantiodetermining step; EXSY, exchange spectroscopy; krel, relative rate constant; ΔGrel, relative Gibbs free energy.
The authors conclude:
This demonstration of the desymmetrization of difluoromethylene groups being achieved by the cooperation of a chiral, low-valent transition metal with a fluorophilic cation could change the role of the difluoromethylene group from an inert unit installed to inhibit metabolism to a prochiral retron for stereogenic alkyl fluorides. We expect that difluoromethylene units in various substructures will react with a range of catalysts and activators when the design elements proposed here are used, thereby expanding the scope of nucleophiles and electrophiles that participate in defluorinative substitution reactions to form alkyl fluorides enantioselectively.
This is very beautiful work, rather important I think, and it's a shame that while humanity is at the precipice of a golden age, fear, ignorance and violence are on the rise.
I trust in spite of all the tragedy we are seeing in the winding down of the awful Trump era, despite the pandemic, that you are taking some time to recall how wonderful it is to be alive.
I wish you safety and good health.





