Science
Related: About this forumToward Sustainable Biologically Derived Anodes for Aluminum Production.
The paper I'll discuss in this post is this one: Synthesis and Characterization of Bio-pitch from Bio-oil (Ying Lu, Dazhi Li, Xianai Huang, Donald Picard, Roozbeh Mollaabbasi, Thierry Ollevier,* and Houshang Alamdari* ACS Sustainable Chem. Eng. 2020, 8, 31, 11772–11782)
The Hall-Heroult process responsible for the production of aluminum metal from alumina, Al2O3, is electrolytic in nature, which theoretically should result in the production of oxygen, but doesn't in practice, do so. In real practice, even though the reaction is most definitely driven electrochemically, the oxidized species does not represent oxide being converted to oxygen gas, but rather represents the oxidation of carbon to give carbon dioxide:
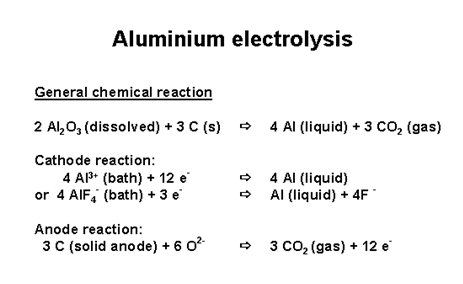
European Carbon and Graphite Association.
Under certain conditions, side products are formed, the greenhouse gas CF4, for example where fluorine source is the flourine gas released from the synthetic cryolite in the molten salt bath, and carbon monoxide, from the Boudouard reaction between carbon dioxide trapped in pores of the anodes and the anode carbon itself. (Other minor gases include tetrafluoroethylene) etc.
According to the information from the World Aluminum Institute the world produced 63,697,000 metric tons of aluminum in 2019. The amount of electricity consumed, also as reported by the World Aluminum Institute to produce aluminum in 2019 was 848,845 GWh. (Accessed 8/17/20) More than half of the world aluminum supply was produced in China - and despite all the bull you can read around here, say over at the E&E forum about how coal is dead - China still burns massive amounts of coal. The World Aluminum Institute, thus reports that of the 848,845 GWh reported to produce aluminum, 509,393 GWh was produced by burning coal. A good working figure for the carbon intensity of electricity produced by coal is 1,100 grams of carbon dioxide are produced for each kwh, meaning that the electricity generated by coal combustion alone in order to drive aluminum plants was 560,000,00 million tons.
This of course, excludes the carbon in the anodes, which are made with coal tar pitch and petroleum coke.
From the equation above, and the atomic weight of aluminum, 28.96 grams per mole, and the molecular weight of carbon dioxide, 44.095 grams per mole, and the ratio - at least at 100% yield (which is unlikely) - of 3 moles of carbon dioxide being produced for 4 moles of aluminum, we can see that 63,697,000 metric tons of carbon dioxide, one can easily calculate that the oxidation of the electrodes added about 27 million tons of carbon dioxide to the atmosphere.
Aluminum production for various technical reasons I will not discuss here, needs to be produced in continuous processes. Although a tiny amount of power was reported for so called "renewable energy" generated electricity by the World Aluminum Institute's 2019 figures, 23,099 GWh, or in the "percent talk" that advocates of so called "renewable energy" love so much, 2.7% of the total electricity produced. Since aluminum plants are required to operate continuously, this 2.7% probably represents almost in its entirety, the Icelandic production using geothermal energy, supplemented by hydroelectricity.
It is not possible to produce reliable continuous power using the much ballyhooed, but entirely ineffective, wind and solar industry, and therefore here, as elsewhere, it is not really possible to displace coal with wind and solar energy, despite so much rhetoric - all of it delusional on a Trumpian scale - to the contrary.
It is, of course, possible to produce continuous power using nuclear energy, which unlike wind and solar can displace nuclear, but despite this fact, nuclear energy contributed only 13,828 GWh to aluminum production, dominated by Europe and China. Hydro is a major player in aluminum production, producing about 40% as much electricity as coal does for this purpose (210,154 GWh) but we are fresh out of rivers to destroy for electricity generation. It does seem that the continual rise in anti-nuke fear and ignorance is slowing down a bit, and may be peaking, but the reality is that nuclear energy has been prevented from reaching its potential by successful appeals to fear and ignorance, and thus aluminum production on this planet is responsible for about 2% of the 35 billion tons of carbon dioxide humanity releases each year.
The point of this diatribe is that the amount of carbon that can be saved using the technology being explored in this paper, the displacement of coal tar pitch with biooil pitch is small, only about 27 million tons, but perhaps a worthwhile object of consideration, since the other metal that uses prodigious amounts of coke is the steel industry. Moreover, considerable amounts of carbon are present in steel alloys, meaning that this carbon in those alloys is sequestered.
Nevertheless, it is important to keep scale in mind when discussing climate change, but too often we don't. This explains why people can prance around obliviously pretending that solar and wind energy matter, when in fact they don't: The failure to appreciate scale.
From the paper's introduction, covering some of the ground I've discussed above:
Biomass is a well-known renewable, sustainable, and environmentally-friendly carbon source. Consequently, it could be a potential alternative source to produce binder for carbon anode making.(4,5) The pyrolysis of biomass can produce solid bio-carbon, liquid bio-oil, and a gas phase, which is the result of chemical reactions involving the molecular breakdown of large molecules into smaller ones at high temperature and in the absence of oxygen. Attributable to the removal of oxygen-rich volatile matters of biomass during the pyrolysis process, the resultant products have a higher heating value.(6)
The "oxygen-rich volatile matters" are probably dominated by methanol, which years ago, when I was a kid, used to be sold in hardware stores as "wood alcohol." Almost all of the methanol now produced on earth is not from the destructive distillation of wood, but rather by the partial oxidation of dangerous natural gas's methane component, or else hydrogenation of carbon dioxide (or monoxide) using hydrogen produced by the reformation of dangerous natural gas.
What the authors explore here is a particular approach to converting "biooil" - the subject of much discussion in the scientific literature - into "biopitch" to be used as a binder to make anodes for Hall-Heroult aluminum production reactors:
The properties are described as such:
"CTP" here is "coal tar pitch."
A brief description of "biooil" is provided:
The process utilized in the paper is vacuum pyrolysis, heating the biooil in a vacuum at different temperatures and for different periods of time.
They purchased the biooil from a supplier. Reportedly the biomass source was largely softwood sawdust, 80% pine, 20% cedar.
The experimental conditions are briefly described as such:
Table 1:

"Coking Value" is a property defined by the fractions extracted into various solvents: Batia [iet al., Journal of Materials Science volume 22, pages3847–3850(1987)]
Some pictures from the text:

The caption:

PAH's (polyaromatic hydrocarbons, aka, PNA (polynuclear hydrocarbons) are the most carcinogenic components of coal tar:
The caption:

The caption:

The caption:
MALDI is "Matrix assisted laser desorption ionization," a mass spectrometry technique for determining the structure of molecules.

The caption:

The caption:

The caption:

The caption:

From the conclusion:
...Finally, a hypothetical reaction mechanism of bio-pitch synthesis from bio-oil was proposed on the basis of the chemical analysis of bio-oil and bio-pitch. The physical properties of the bio-pitch were characterized and compared to those of coal-tar-pitch. Compared to coal-tar-pitch, all bio-pitch samples exhibited much lower PAHs, quinoline insolubles, and sulfur contents, representing significant health and environmental advantages as a binder in the anode formulation. It was shown that some distillation conditions, i.e., temperature, heating rate, and pressure, are important parameters to adjust the softening point of the bio-pitch and its viscosity, both being important anode manufacturing parameters. Further investigation is required to evaluate other characteristics of bio-pitch in order to develop a new and environmentally-friendly alternative binder in the anode formulation and to confirm its appropriateness for replacing coal-tar-pitch...
From my perspective, an optimal approach to removing carbon dioxide from the air will depend on having an economic incentive for doing so, the key to this being useful materials. Coupled with the only sustainable form of energy that exists, nuclear energy, it seems remotely possible - although by no means certain - that we can reverse the indifference and self delusion that have led to the destruction of the planetary atmosphere. Some of it will involve little steps, like anodes from biomass, and some other large steps, like displacing all dangerous fossil fuel plants with nuclear plants, rendering so called "renewable energy" - which is decidedly not sustainable precisely because of its mass requirements - unnecessary and superfluous.
These ideas of mine are, of course, not popular, but I have convinced myself it would be morally unacceptable, for me personally at least, not to state them.
This little paper made me quite happy, since the issue of anodes has been troubling me for some time, given my fondness for metallurgy and my excitement over the new vistas opened by the Cambridge FFC process, and electrolytic process similar to that of the Hall-Heroult process long in use for aluminum.
I hope and trust that even in the age of Covid, some part of your summer has been pleasant and rewarding.
hunter
(40,479 posts)Fluoride pollution from aluminum smelters is a problem everywhere, especially in nations without adequate environmental protections. Aluminium smelting is the largest single producer of toxic fluoride pollution worldwide.
From what I gathered in a quick internet search the FFC process is currently used for higher value metals.
NNadir
(37,550 posts)To some extent, the Hall Heroult process is an electrochemically driven carbothermic reduction.
The FFC Cambridge process is an electrochemically driven calcium metal reduction.
I do believe it's cleaner; not without problems of its own, but cleaner.
We'll certainly see. The original FFC process target was titanium; and titanium is potentially a wonderful industrial metal for which - as was historically the case with aluminum until Hall-Heroult - a cheap way to reduce TiO2 in its allotropes to the metal was the main problem, particularly because the Kroll process is difficult to make continuous.
