Science
Related: About this forumSpeculations on Covid-19 Vaccine Production Bottlenecks.
The paper I'll discuss in this post is this one: Optimization of Lipid Nanoparticle Formulations for mRNA Delivery in Vivo with Fractional Factorial and Definitive Screening Designs (Kevin J. Kauffman, J. Robert Dorkin, Jung H. Yang, Michael W. Heartlein, Frank DeRosa, Faryal F. Mir, Owen S. Fenton, and Daniel G. Anderson
Nano Letters 2015 15 (11), 7300-7306)
Recently on this website, I described how I cheered up my doctor by relaying to him "war stories" from the scale up of HIV protease inhibitors in the 1990s, of which I am a "veteran" of sorts. It's here: I hope I cheered my doctor up.
This morning I attended a (Zoom) lecture by an astrophysicist at Princeton University Professor Cristiano Galbiaiti who works on neutrino and dark matter detectors which rely on highly purified gases, who used his expertise and knowledge of them to rapidly design a new ventilator that was cheap, easy to manufacture, and proved to be highly effective for the treatment of Covid-19. It obtained the fastest approval, from conception to patient use, of a new medical device by the USFDA, 45 days.
It is an example of how "impractical science," the discovery of neutrinos in space, allows for "practical" innovation, much as the 1960's space program allowed for the development of small portable computers, on which many of us now rely.
If you member, in the early days of Covid, there was a huge shortage of ventilators, and the Canadian government, which funded the design and manufacture of the ventilators, ordered many tens of thousands of these new ventilators, which were delivered. The end result is interesting: There is now an oversupply of them, and the partners working on this project are now working to get Western governments to donate ventilators to Africa, where, predictably, ventilators are still in short supply.
I have to see my doctor again this week, and I decided to look into the manufacturing process of RNA vaccines, so I could further cheer my doctor up, with some more detailed insights into the manufacture of these vaccines, about which I have a sense of regulatory requirements, but not the actual details.
I am not currently in anyway involved in the manufacture of Covid vaccines, but I have had professional conversations about nucleic acid formulations, and have had other conversations relating to, and sometimes supporting, related "lipid nanoparticle" work, notably liposomes. Anything I say in this post will, however, be largely guesswork and should be taken with a grain of salt.
The HIV protease inhibitors are drugs that are considered "peptidomimetics" and they depended on access to certain amino acids as starting materials, in particular cysteine and phenylalanine (depending on the protease inhibitor in question). Since many chemical synthesis steps were involved in converting them into "isosteres" of peptides, these starting materials were unregulated commodity items required only to meet certain specifications subject to somewhat limited specifications.
What was involved was large chemical reactors, performing organic synthesis on a multi-ton scale, in some cases utilizing fairly dangerous reagents. (Phosgene was one.) Ultimately these were utilized to make the "API" - the active pharmaceutical ingredient, the protease inhibitor.
The situation with respect to the API of nucleic acid drugs is somewhat different, I expect.
Nucleic acids, such as DNA and RNA, are self replicating molecules of course, and so in theory, their production can (and does) proceed exponentially. This is the technology behind forensic and commercial (as in "23 and me" and related companies) "PCR" (Polymerase Chain Reaction) technology. It is the design of the nucleic acid that matters.
A nice overview of the design and some information about production of the API can be found in an open source article that is somewhat, but not, I think, overly, technical: The promise of mRNA vaccines: a biotech and industrial perspective. A cool feature of the design of these vaccines, with which I was unfamiliar, is the realization that they not only encode for the immunogen protein of the SARS-CoV-2 virus, the famous "spike" protein, but they also encode for some proteins designed to aid with the transcription of the RNA to generate the proteins.
I really can't say very much intelligent about the scale up of the API and potential bottlenecks, since these are involved more with biological systems than synthetic systems and I personally have far less exposure (mainly osmotic exposure) to these than I do to synthetic systems. I would imagine that the chief bottleneck might be having useful enzymes for transcription from the DNA parent the RNA, as well as nucleic acids themselves, although, as opposed to amino acids, which in living systems have 20 basic components (actually a few more because of "post translational modifications" ) there are only 8 nucleic acids in living cells, making separations more straight forward. I would imagine (but do not know) that these are synthetically available without isolation from natural sources (as are some amino acids) from ribose, deoxyribose (almost certainly obtained by fermentation) and the nucleobases which are readily available commodities lacking stereocenters. I know for a fact, that enzymes, including certainly the enzymes responsible for nucleic acid transcription are readily available from biological fermentation facilities. It is possible there has been some strain on these supplies, but basically a vast infrastructure exists.
So my guess is that the API's are not much of a bottleneck at all, beyond, it would seem, some industrial scale chromatography in at least some steps; I would imagine, perhaps, affinity chromatography, which is much, much cleaner than other types of chromatography, perhaps even at the level of solid phase affinity extraction. (These are all speculations; I have no information.)
My guess is that the real issue, the biggest bottleneck, is the formulating agents.
Nucleic acids are charged species, hydrophilic species, that is they are soluble in water and other polar solvents. All cell membranes, by contrast, are lipids, aka "fats." The basic rule that "like dissolves like" applies, which is why cells like blood cells are able to stay intact; their membranes are insoluble in water. Therefore the trick is to get the charged nucleic acids through the membrane, and into the cell. This requires amphiphilic lipids, which have hydrophobic properties on one end of the molecule, and hydrophilic properties on another end. The most famous of these are soaps, with which everyone is familiar.
In fact, the SARS-CoV-2 virus is coated with a lipid, and this is why soap, regular hand soap, is so effective at inactivating it, since it strips away the lipid layer on which the virus depends.
Fats are readily available from multiple sources, so much so that they have been utilized as fuels, biodiesel, in automobiles and trucks by the so called "renewable energy" industry, which has succeeded in destroying large swathes of Asian rain forest for palm oil plantations in part to make diesel fuel for German cars and trucks.
I want to be clear on something in this context, since I am about to discuss lipids, the amount of palm oil required to make the world supply of Covid vaccines (if palm oil is a basic source at all, which it may not be) will surely be trivial when compared to produce other commodities, including biodiesel and the palm oil utilized in foods. There is really no legitimate rationale for destroying the South Asian rain forests for commercial interests. The very special lipids that are likely utilized in Covid vaccines might have just as easily utilized corn oil as palm oil, since it is likely that the starting materials, saturated and unsaturated straight chain fatty acids are present in both sources, and perhaps other temperate zone agricultural products such as canola oil or soybean oil.
Anyway.
The lipids in question are amphiphilic, but they are very special lipids of a type, which are widely found in living systems, but which are also synthetically complex except by enzymatic routes.
To give a feel for the complexity of lipids beyond the straight chain fatty acids, here is a chart showing types of a class of lipids that are found prominently in skin, the ceramides:
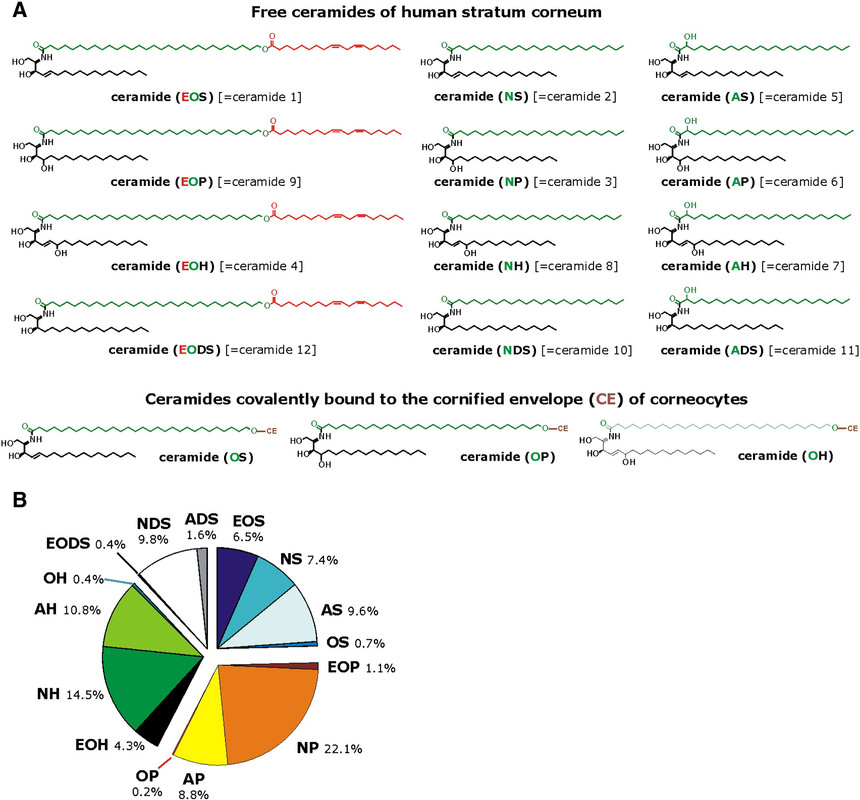
Some of the lipids described in the paper cited at the beginning of this post are isosteric (similarly shaped) to sphingosines.
The key point in this development is the concept of "ionizable lipids," which address the transport of a charged species, RNA, across the lipid membranes of cells.
(At this point, it behooves me to mention a post in this forum by our leader, pointing to the career of a famous lipid chemist in the early days of lipid chemistry: Watch for NOVA's rerun of "Forgotten Genius" in your area ... or watch it online ...
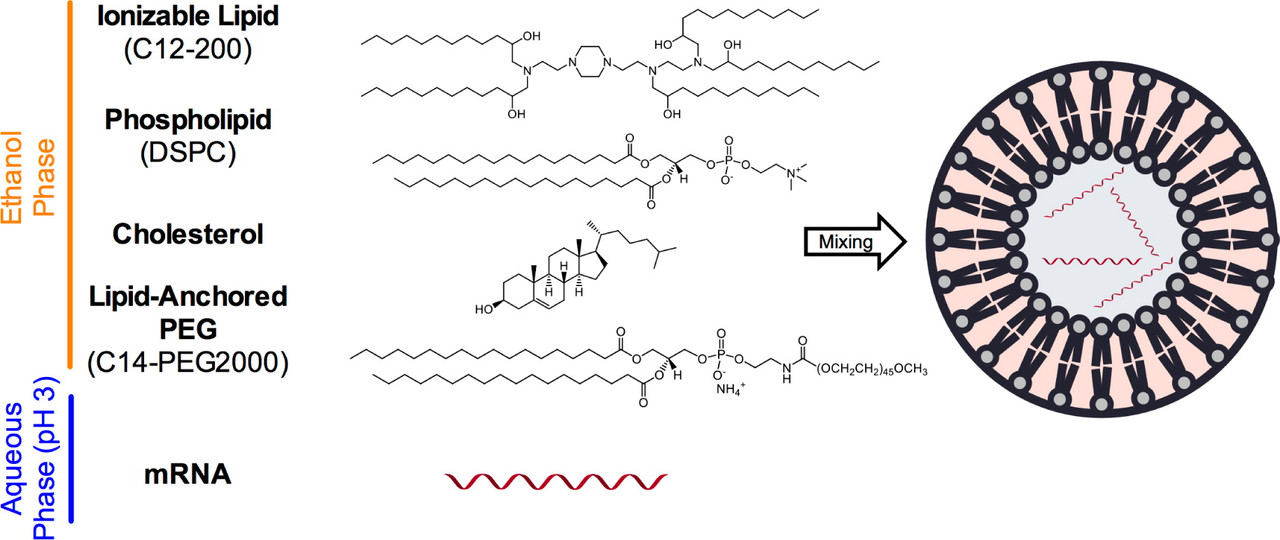
From the introduction to the paper cited at the outset:
In addition to the ionizable material, three other excipients are also commonly used to formulate LNPs: (1) a phospholipid, which provides structure to the LNP bilayer and also may aid in endosomal escape; (2, 13) (2) cholesterol, which enhances LNP stability and promotes membrane fusion; (14, 15) and (3) lipid-anchored polyethylene glycol (PEG), which reduces LNP aggregation and “shields” the LNP from nonspecific endocytosis by immune cells.(16) The particular composition of the LNP can also have profound effects on the potency of the formulation in vivo. Several previous efforts to study the effect of formulation parameters on siRNA-LNP potency utilized the one-variable-at-a-time method,(17, 18) in which formulation parameters were individually varied to maximize LNP potency; this approach, however, does not allow for examination of potentially important second-order interactions between parameters. Inspired by statistical methodologies commonly used in the engineering and combinatorial chemistry literature,(19, 20) we chose to utilize Design of Experiment (DOE) to better optimize LNP formulations for nucleic acid delivery. Using DOE, the number of individual experiments required to establish statistically significant trends in a large multidimensional design space are considerably reduced, which is particularly relevant for the economical screening of LNP formulations: in vitro screens are often poor predictors of in vivo efficacy with siRNA-LNPs,(21) and it would be both cost- and material-prohibitive to test large libraries of LNP formulations in vivo.
To demonstrate the application of DOE to LNP formulation optimization in vivo, we formulated LNPs with a different type of nucleic acid than siRNA. Recently, messenger mRNA (mRNA) has been investigated for therapeutic protein production in vivo, including applications in cancer immunotherapy, infectious disease vaccines, and protein replacement therapy.(22, 23) Unlike plasmid DNA, mRNA need only access the cytoplasm rather than the nucleus to enable protein translation and has no risk of inducing mutation through integration into the genome.(24) Because there are inherent chemical and structural differences between mRNA and siRNA in terms of length, stability, and charge density of the nucleic acid,(25) we hypothesized that LNP delivery formulations for mRNA may require significant variation from those developed for siRNA delivery...
The authors developed a combinatorial approach using chemical libraries (compounds modified and mixed in a controllable but different way) to screen lipid formulations.
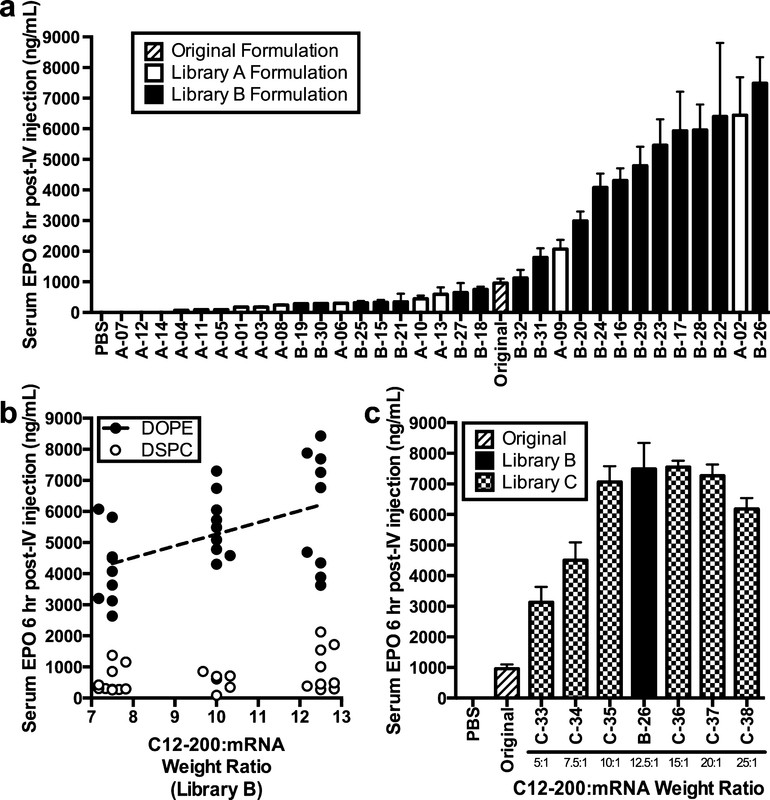
There are many hundreds of papers on the topic of lipid particles utilized in nucleic acid drug development, but for this brief post, we'll just look at the pictures produced here, and return to figure one for further discussion:
The caption:
The caption:
EPO here, is one of the early protein drugs on the market, one that got Lance Armstrong and other athletes busted, the anti-anemia drug erythropoietin, which jacks up hemoglobulin in the blood. The idea here is to insert RNA into cells in such a way that rather than being produced in a reactor, it is produced within the blood itself. (I would imagine that tracing this would make anti-doping efforts somewhat more challenging.
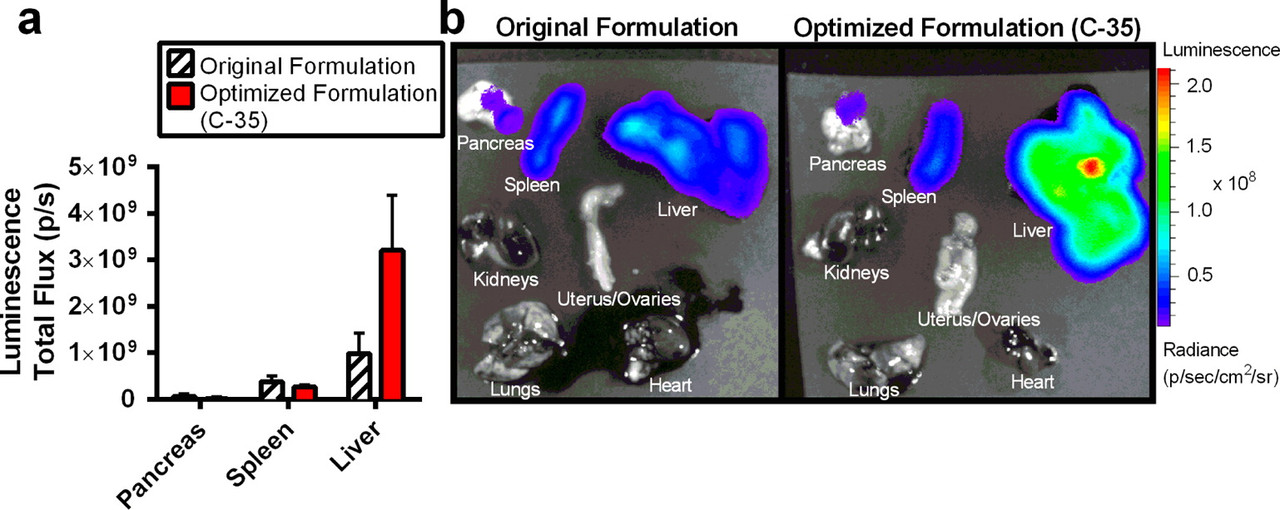
The caption:
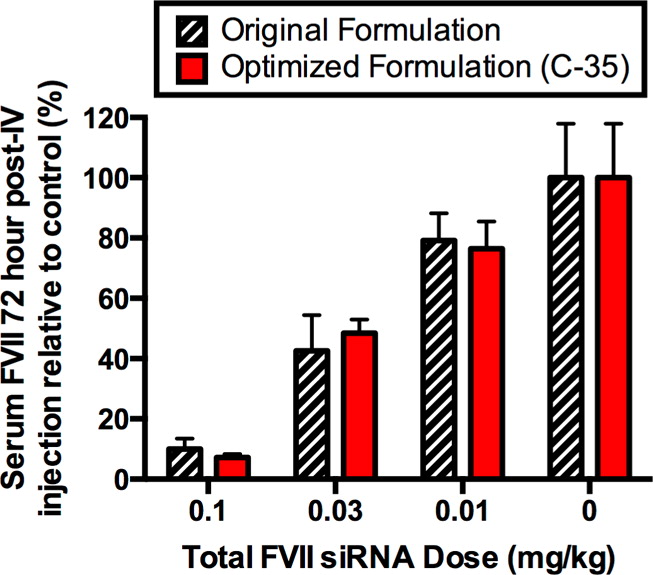
The caption:
The experimental portion of the paper, by the way, describes the synthesis of the mRNA itself that was used in this study; it is very "PCR" like, an exponential growth system. Certainly some clean up is involved, but I suspect this is not a bottle neck.
mRNA was synthesized by in vitro transcription from a plasmid DNA template encoding the gene, which was followed by the addition of a 5′ cap structure (Cap 1) using a vaccinia virus-based guanylyl transferase system. A poly(A) tail of approximately 300 nucleotides was incorporated via enzymatic addition employing poly-A polymerase. Fixed 5′ and 3′ untranslated regions were constructed to flank the coding sequences of the mRNA.
Now I'ld like to return to figure 1, which I repeat for conveninence, and make some comments.

Without having sourced it myself, I would imagine that cholesterol is easy to get from a variety of sources.
What are interesting are the other three molecules (which may represent classes of molecules in real cases or similar cases).
These I would imagine (and in one case, I know) are chemically synthesized in chemical reactors.
First the "ionizable lipid." There is an element of pseudosymmetry in this molecule, inasmuch as all the long carbon chains are C12, and are almost certainly obtained from lauric acid, the C12 fatty acid, which I know from experience is available in high quality from a number of sources. The core of the molecule is piperazine, the cyclic molecule in the center with two nitrogen atoms in the ring on opposite sides, also a commercially available reagent. Nevertheless, there is symmetry breaking inasmuch as the spacers in the two nitrogens on the piperazine ring have different spacers. This means to make the molecule, one must protect one (and only one) of the nitrogens, which is not entirely straight forward, or otherwise synthesize the ring in such a way that one nitrogen is protected, eliminating the advantage of using very cheap and readily available piperazine. My organic synthesis muscles are no longer strong enough to offer a route off the top of my head to the OH group that is one carbon removed from the amino nitrogens in those side chains, but I'm quite sure if one looks, one can find such a route.
Nevertheless, although very large reactors for making this "ionizable lipid" are certainly available under regulated ("GMP" ) conditions, this would be a bottleneck, if this particular (or a similar) "ionizable lipid" were utilized in formulating either the Moderna or the Pfizer vaccines.
The DSPC is a derivative of phosphocholine, which is found in many foods, notably eggs, and is fact found all over living organisms. In theory it can be made from one of the cheapest industrial chemicals there is, glycerol, a side product of the soap and biodiesel industries which is so cheap that people often dump it rather than sell it if they are far from a facility that uses it. The DSPC is a chiral molecule exhibiting "handedness" (being non-superimposable, like right and left hands). I'm sure that there are lots of enzymatic routes to making phosphocholine, but still, after one has it, one has to acylate the two oxygens with steric acid, also a commodity produced from things like palm oil, corn oil, canola oil...etc...etc. Still one needs a reactor to do this.
Finally there is there is "lipid anchored" "PEG," polyethylene glycol, which is also a derivative of phosphocholine, in fact, it is a derivative of DSPC. It is the PEG side chain that matters. The thing that disturbs me a little bit is the designation, at least in this paper, of a fixed length. PEG in most drugs - it is commonly used in many protein drugs to prevent their rapid breakdown in the body - is a polymer, and generally has a molecular weight distribution centered around a specific molecular weight, and also containing some molecules of higher and some of lower molecular weight. It is generally made by the polymerization of ethylene oxide (oxirane). I would hope that the formulations of the lipids in the vaccines do not actually in real life involve this restriction, since this would be problematic and represent a real bottle neck.
But even if it was...
One of the largest selling drugs in the world for many, many, many years was atorvastatin (Lipitor). It was discovered and initially developed at a company that no longer exists, Parke Davis. Here is the structure of atorvastin:
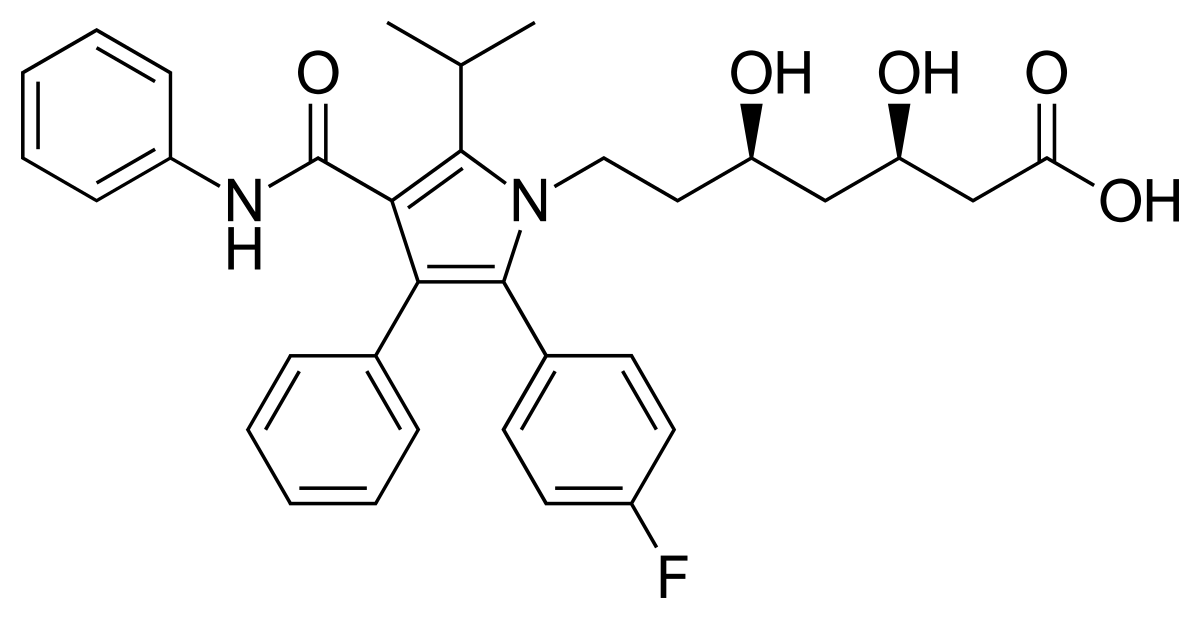
I was told a story about atorvastatin that I heard; it may or not be true. This drug was developed in the 1980's when industrial chiral synthesis was still somewhat problematic - it was a problem generally solved by the time HIV protease inhibitors came around - but early on it wasn't. I was told that Park Davis felt that atorvastatin would never be a big seller, because it was too expensive to make on an industrial scale, because of the seven carbon side chain with the two hydroxygroups on it, both of which are present as chiral centers. In the early days of scale up of the drug, the cost of the side chain was said to be over $1000/kg in 1980s dollars. I know people who were selling it for $800/kg in the 1990s.
Every chiral organic chemist in the world, both industrial and academic, looked at that side chain and figured that they could make a killing by making it cheaper; everybody in the world was going to get rich making it for $500/kg. Companies were founded on this expectation.
So many people exercised their genius to make the molecule cheaper that it ultimately became a commodity, and everybody was trying to undercut everyone else, because everyone's cheaper route made for an oversupply. I heard, that the cost of the side chain, before the expiry of the Park Davis successor company(ies) (ultimately Pfizer) the side chain was priced well under $100/kg, I heard $50, out of India and China.
So what's the story, morning glory?
Yeah, right now, there are bottlenecks, but they aren't going to last very long. Within a year, maybe sooner, these vaccines are going to be commodities, because, right now, there is clearly intellectual and almost certainly physical infrastructure to do it.
And that's a good thing. In the end, it will come down to logistics and little else.
Cheer up. We have a smart and energetic President who actually gives more than a rat's ass, vastly more than a rat's ass, as opposed to his orange predecessor, and we will beat this thing.
Some suggested further reading:
Martin A Maier, Muthusamy Jayaraman, Shigeo Matsuda, Ju Liu, Scott Barros, William Querbes, Ying K Tam, Steven M Ansell, Varun Kumar, June Qin, Xuemei Zhang, Qianfan Wang, Sue Panesar, Renta Hutabarat, Mary Carioto, Julia Hettinger, Pachamuthu Kandasamy, David Butler, Kallanthottathil G Rajeev, Bo Pang, Klaus Charisse, Kevin Fitzgerald, Barbara L Mui, Xinyao Du, Pieter Cullis, Thomas D Madden, Michael J Hope, Muthiah Manoharan, Akin Akinc, Biodegradable Lipids Enabling Rapidly Eliminated Lipid Nanoparticles for Systemic Delivery of RNAi Therapeutics, Molecular Therapy, Volume 21, Issue 8, 2013,
Dynamic PolyConjugates for targeted in vivo delivery of siRNA to hepatocytes
David B. Rozema, David L. Lewis, Darren H. Wakefield, So C. Wong, Jason J. Klein, Paula L. Roesch, Stephanie L. Bertin, Tom W. Reppen, Qili Chu, Andrei V. Blokhin, James E. Hagstrom, Jon A. Wolff, Proceedings of the National Academy of Sciences Aug 2007, 104 (32) 12982-12987; DOI: 10.1073/pnas.0703778104
Semple, S., Akinc, A., Chen, J. et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 28, 172–176 (2010). https://doi.org/10.1038/nbt.1602
Many, many, many more papers may be found by calling these papers up in Google Scholar, and clicking on the citations list.
I hope you will have a safe and enjoyable Sunday.