Science
Related: About this forumElectrolysis of Lithium-Free Molten Carbonates
The paper I'll discuss in this post is this one: Electrolysis of Lithium-Free Molten Carbonates (Xiang Chen, Zhuqing Zhao, Jiakang Qu, Beilei Zhang, Xueyong Ding, Yunfeng Geng, Hongwei Xie, Dihua Wang, and Huayi Yin ACS Sustainable Chemistry & Engineering 2021 9 (11), 4167-4174)
I've argued before in this space that electricity is a thermodynamically degraded form of energy: Synthesizing Clean Transportation Fuels from CO2 Will at Least Quintuple the Demand for Electricity.
I have also argued, sometimes while addressing the stupid but oft discussed solar thermal energy fantasy, that the thermochemical splitting of carbon dioxide into carbon monoxide - from which pretty much any industrially produced carbon compound can be made (just add water) - and oxygen is the most efficient approach to carbon dioxide reduction: Cerium Requirements to Split One Billion Tons of Carbon Dioxide, the Nuclear v Solar Thermal cases.
Although for certain reasons I am very fond of the cerium based thermochemical splitting of carbon dioxide, many other such catalytic systems are known for this highly endothermic reaction.
The international symbol for dangerous fossil fuel free energy has become a wind turbine or solar cell graphic object, which is frankly as silly as waving a graphic of a Roman executioner's cross as a symbol for the way to address immorality; both are faith based. It has been experimentally verified, at a cost of trillions of dollars, that wind turbines and solar cells are not even remotely capable of addressing climate change. In fact, the worship of them - and let's be clear that it's nothing more than faith based worship - has led to the acceleration of climate change. The average concentration of the dangerous fossil fuel waste carbon dioxide in the atmosphere measured at the Mauna Loa Carbon Dioxide Observatory during the week beginning on March 19, 2000 was 370.98 ppm. This morning, the latest figure was 417.67 ppm. The current 12 month running average of weekly measured increases over carbon dioxide increases over the last ten years is 24.24 ppm, 2.42 ppm/year. The same 12 moth running average for the week beginning March 19, 2000 was 15.14 ppm, 1.51 ppm/year.
These are facts. Facts matter.
Heckuva job humanity at addressing climate change with all those wind turbines, solar cells and electric cars, heckuva job.
The graphic attached to the introduction of this paper is nonetheless the equivalent of the Roman execution's cross as applied to so called "green" energy. Here it is:

What I like about this paper is that it is cognizant of the fact that matter, specifically the individual elements in the periodic table, is not "renewable." The claim that we can save the world with batteries is no different than the claim that supplies of dangerous petroleum, dangerous natural gas, and dangerous coal are infinite, as well as the claim that places to put the waste, chiefly, but hardly limited to, carbon dioxide is unlimited.
They start out talking about lithium and then consider elements that are far more available, one of which, strontium, catches my eye. Overall this discussion of batteries clearly has some relevance to the "batteries will save us" fantasy.
From the introduction to the paper:
They have a nice graphic on the point:
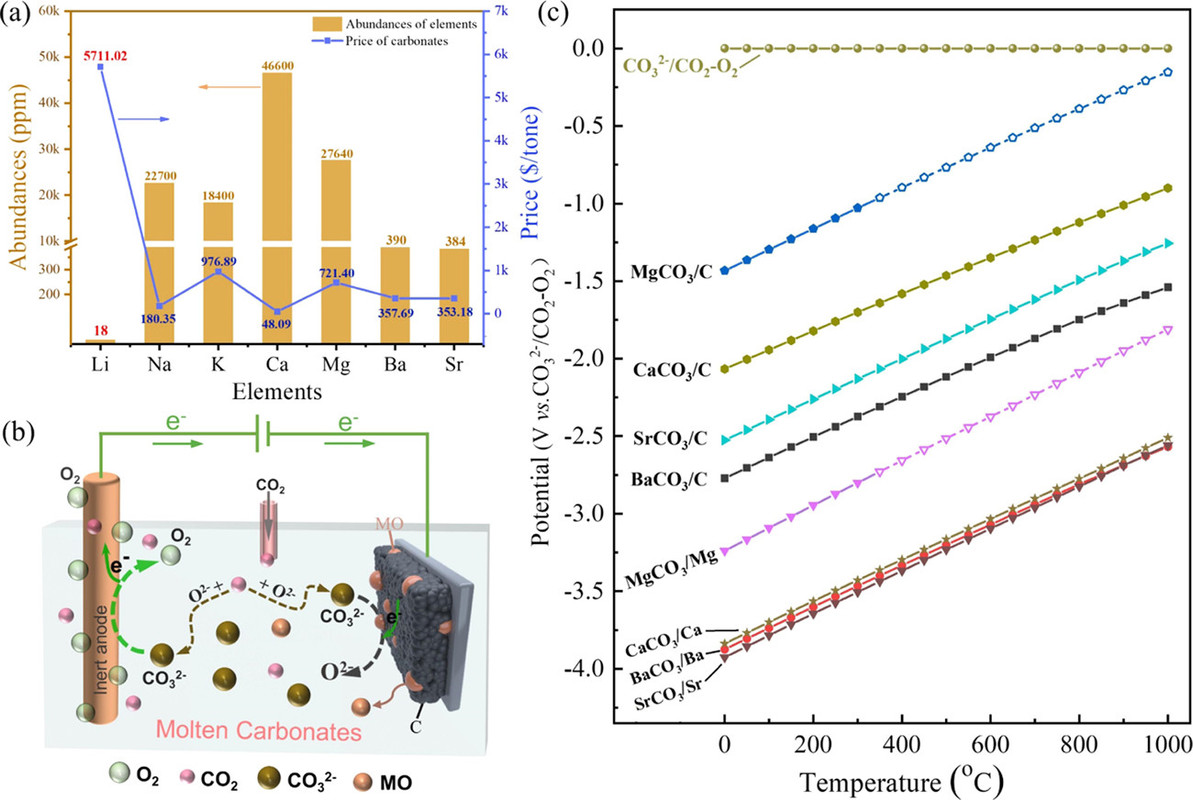
The caption:
They continue:
The use of molten calcium salts is the essence of the FFC Cambridge process for the electrochemical reduction of metals, which I personally believe should be world changing, even if it is true that electricity is a thermodynamically degraded form of energy.
In saying this, they do raise some issues to be addressed:
These factors are what they examine in the paper, and then propose an electrode to reduce carbon dioxide to carbon, in effect reversing the combustion of the dangerous fossil fuel coal.
This process, which requires the input of a thermodynamically degraded form of energy, electricity, as well as heat suggests an important point that is often over looked, which is this: In order to capture carbon dioxide in air one needs to put more energy into the system than all of the original energy ever released by the original combustion of the dangerous fossil fuel produced. This reality should sober up all the drunken handwaving and wishful thinking that goes on when energy and the environment is discussed, but I confidently predict, on the basis of being a tired old man, that it won't.
Their important concern is thermodynamics:
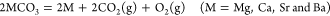 (1)
(1)
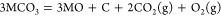 (2)
(2)
If the MO in eq 2 is soluble, then the carbonization reaction between MO and CO2 spontaneously occurs and forms fresh carbonate to maintain the concentration of CO32– of the electrolyte:
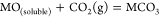 (3)
(3)
The overall reaction of eqs 2 and 3 is
 (4)
(4)
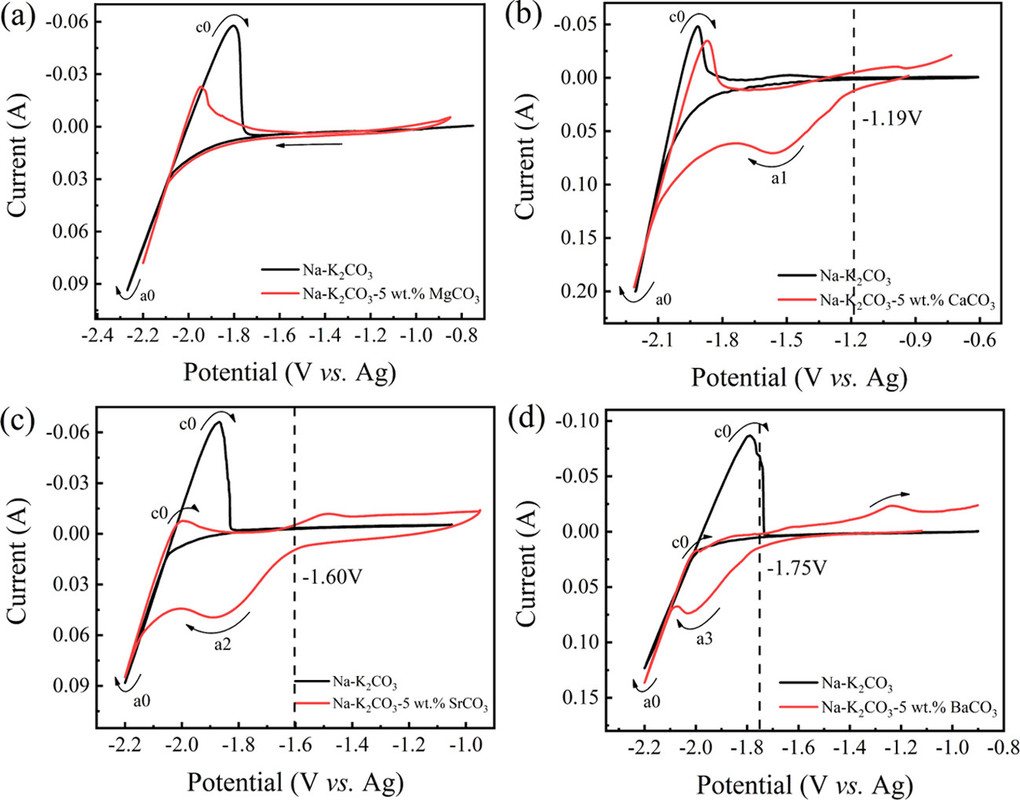
Thermodynamically, alkaline-earth metal carbonates (MCO3, M = Mg, Ca, Sr, and Ba) can be electrochemically converted to carbon at the potential prior to the deposition of the alkaline-earth metal (Figure 1c). Therefore, the deposition of carbon is thermodynamically easier than that of metal. Moreover, the thermodynamic favorability of generating carbon is different depending on the different alkaline-earth metal cations. For example, the thermodynamic deposition potential sequence follows CaCO3 < SrCO3 < BaCO3. In other words, CaCO3 can be reduced to carbon at the potential more positive than that of SrCO3 and BaCO3. Note that the potentials of carbon generation in Na2CO3 and K2CO3 are more negative than that of the deposition of the corresponding alkali metals. Thus, Na2CO3 and K2CO3 are usually employed as the supporting electrolyte.(39,44) Therefore, alkaline-earth carbonates are the solute to be reduced for the carbon generation in MCO3–Na2CO3–K2CO3 (M = Mg, Ca, Sr, and Ba).
The reduction behaviors at a Mo electrode in a variety of carbonates containing different alkaline-earth metals are studied. As shown in Figure 2a, no reduction peaks were observed before the cathodic limit in the pure molten Na2CO3–K2CO3, demonstrating that Mo is an inert material that does not involve in any electrochemical reactions in the selective potential range.
The preliminary experiments investigated the cyclic voltammograms of molten carbonate systems using molybdenum electrodes:
The caption:
Carbon was indeed deposited under these conditions, at a temperature of 750°C in sodium/potassium carbonate melts containing 10% (by weight, surprisingly) of three alkali metal carbonates, those of calcium (as in the FFC Cambridge Process), strontium and barium.
Micrographs of the carbons formed are shown:
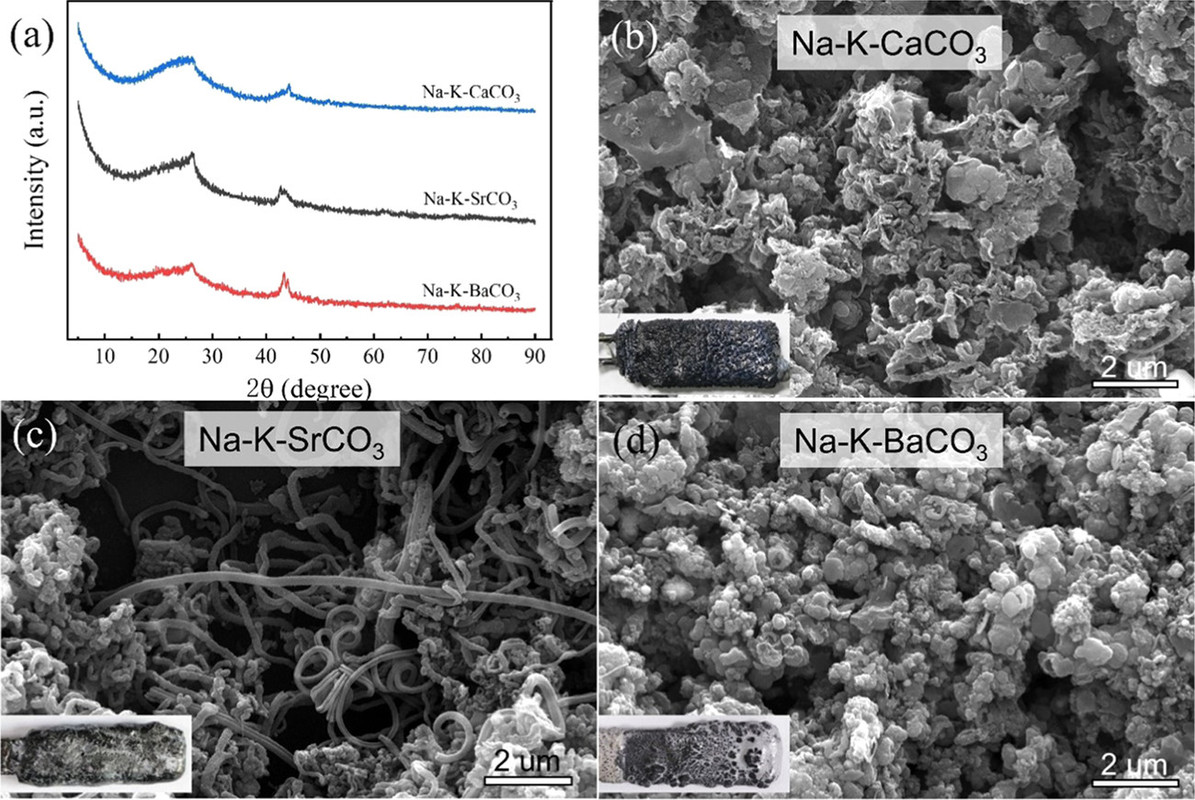
The caption:
The question next turned to finding a stable electrode for the oxidation side of the reaction:
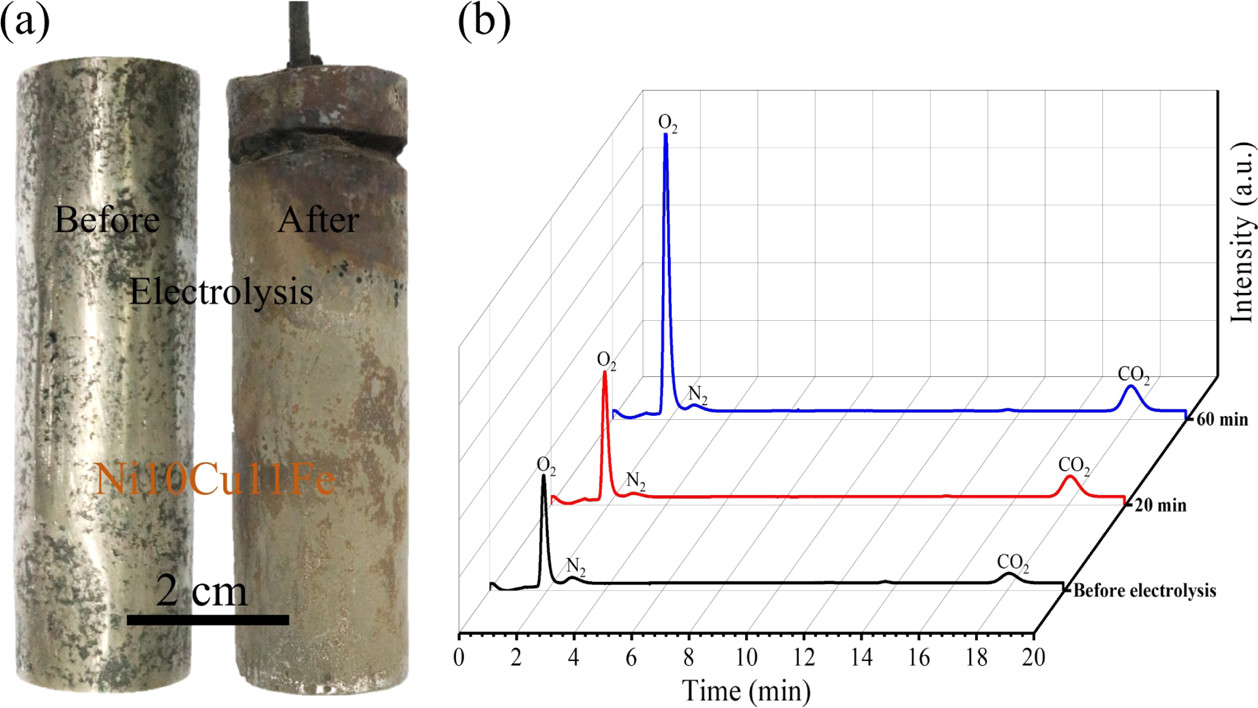
The caption:
Next the solubility of the various oxides, calcium, strontium, and barium were determined by the simple expedient of putting pellets of these oxides in the melts and observing the amount dissolved:
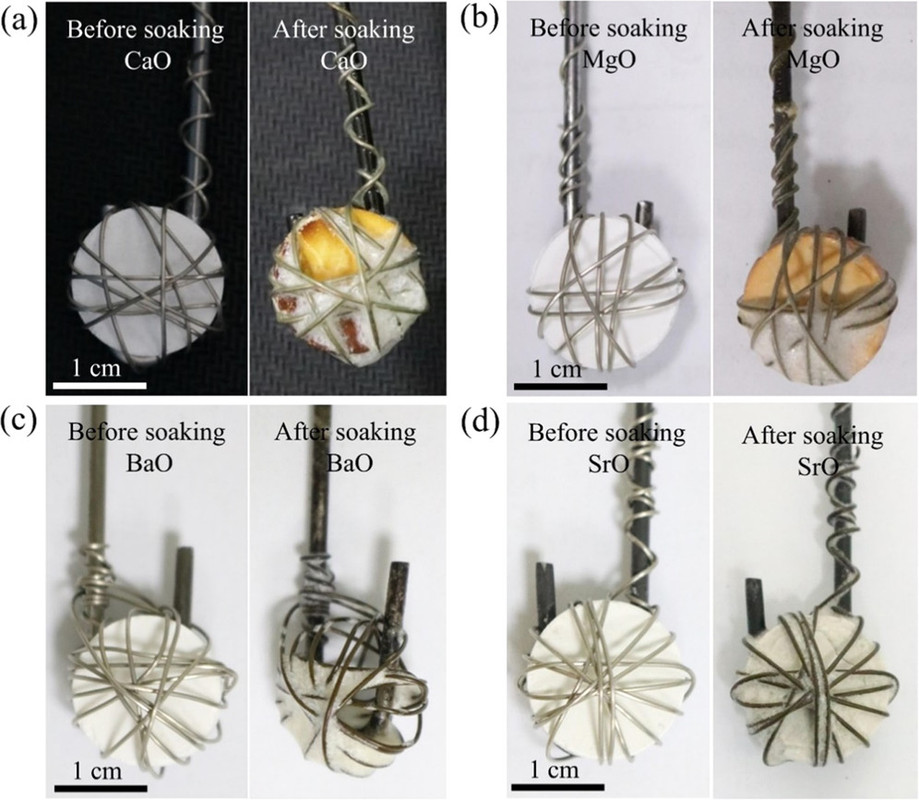
The caption:
Quantitative tables of the solubility of these oxides are not given in the paper, but the general conclusion was that barium oxide was the most soluble and the solubility of its oxide and carbonate were evaluated in more detail along with the rate of dissolution, as shown in the following graphic:
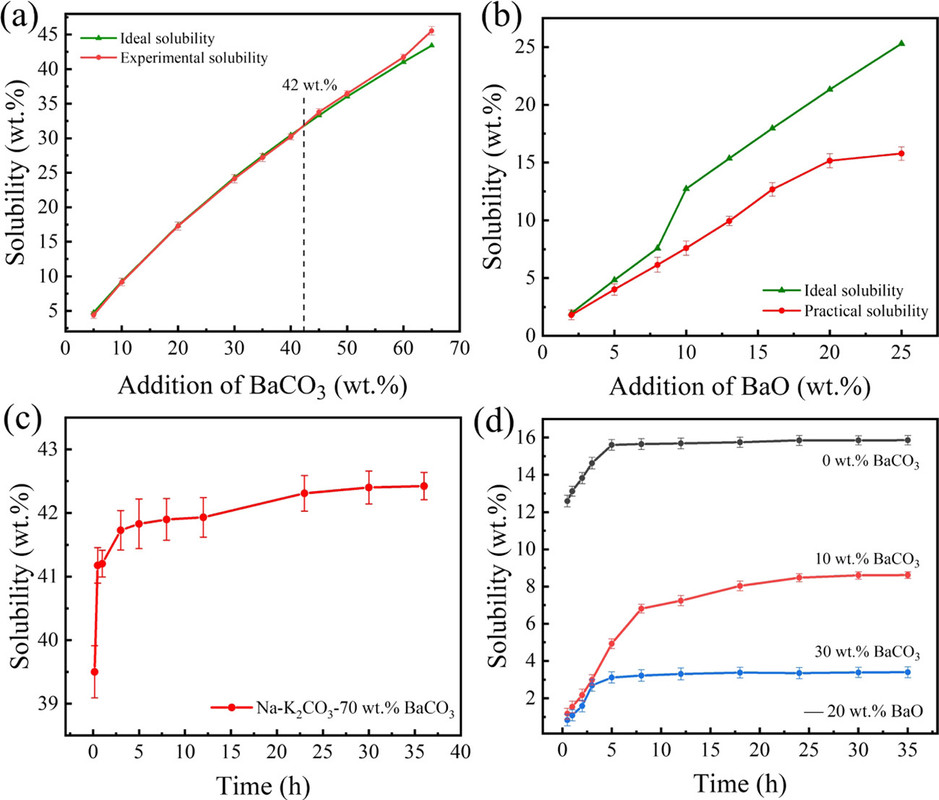
The caption:
It would be nice, from my perspective, if a more complex carbonate system allowing for the use of strontium in this system were considered in subsequent optimization, since strontium is available from used nuclear fuel with a heat generating isotope, Sr-90, which might defray the environmental and economic cost of maintaining a melt, although there are many heat network setting which might address this problem at an acceptable environmental and economic cost.
In any case, the barium oxide can absorb gaseous oxygen, even at these high temperatures - the decomposition temperate of barium carbonate is around 1300°C.
If the goal is, however, to produce the oxides themselves of strontium and/or calcium - the latter oxide is a key constituent of concrete, concrete production being a major contributor to climate change, the insolubility of the oxides is actually a desirable outcome.
The authors write:
This figure obviates that point:
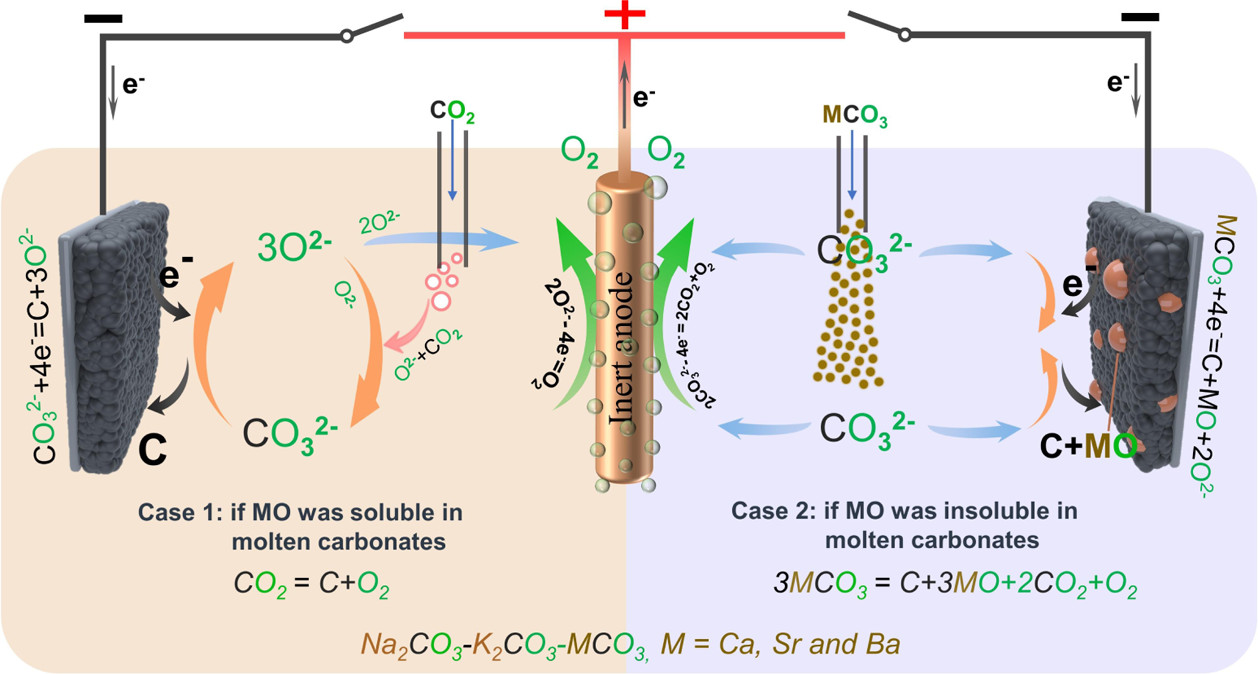
The caption:
They then write about capturing carbon dioxide from flue gas; if this gas is formed to generate electricity, they are then talking about a perpetual motion machine, but that doesn't totally negate the value of the paper itself.
I began this commentary by noting that electricity is a thermally degraded form of energy, but did not state the caveat that electricity captured by use of waste heat that would otherwise be rejected to the atmosphere is merely an improvement in overall energy efficiency. In the case where electricity is generated as a side product, using waste heat from a very high temperature process, the second law of thermodynamics is still operative - there is no physical way to make it inoperative - and increased exergy is obtained.
The conceit surrounding the so called "renewable energy" fantasy is that the intermittent nature of electricity can be addressed by net metering. On the left, we like to mock the statements of the assholes running ERCOT and the racist governor of Texas that the recent events in that State that led Senator Cancun Cruz to run away, the collapse of its power system was an outgrown of the so called "Green New Deal." To be perfectly clear and unambiguous, even as a lifelong Democrat, I don't think that the "Green New Deal" is going to be green or much of a deal. I have zero respect for the tired and old, and frankly experimentally failed energy ideas of Ed Markey, whether I otherwise agree with his other politics, as I often do. So called "renewable energy" is failing us now, and worse, is failing the future. It didn't work to address climate change. It isn't working to address climate change. It won't work to address climate change. Period. A key concept in the ideology of Green New Dealism is so called "net metering" which is the idea that the use of electricity follows its availability, its availability determining its price. If we are honest with ourselves, not that it is easy for anyone to be honest with themselves when honest confronts faith, including quasi religious faith, even absurd faiths, the extreme utility bills seen by some citizens of Texas are "net metering" run wild.
A heat network run by a high temperature nuclear plant, one producing temperatures high enough thermochemically split carbon dioxide - the cerium cycle running at maximum temperatures of 1400°C - will achieve the highest efficiency - the yield of exergy - as a constituent of a heat network, possibly producing a number of Brayton, Rankine and even Sterling cycles in sequence. If this is the case, electricity may be inevitably a side product. If this electricity is used to run combustion in reverse, which is what this paper is all about, the electricity can be switched to the grid whenever electricity prices rise high enough to justify the switching. In effect, a chemical plant is run as spinning reserve. This is not a new idea. Kaiser aluminum - aluminum production is an chemoelectric process - did this historically with hydro power in the Pacific Northwest.
Power demand on the grid fluctuates regularly, with the highest demand in the late afternoon and early evening, precisely when the sun goes down ironically enough, since so many people actually believe that solar electricity will save the world. It won't. It hasn't. Exploiting these fluctuations can be utilized to produce molecular carbon from CO2 gas or provide electricity to the grid, depending entirely on the value to the operator of the plant.
From the paper's conclusion:
It's a very nice paper. I like it a lot.
I trust you are enjoying your weekend.
