Science
Related: About this forumListen guys, I hate to tell you this, but you've got missing planes.
I always love to read the part of Journals, almost always at the end of an issue, where one scientist or group offers a commentary on an earlier paper and receives a response from the original authors commenting on the comments made. Sometimes these exchanges can border on hostility, but at other times, it can be an enlightening exchange.
Such an exchange took place in the current issue of Nature as of this writing. It's an article about lead perovskites, which are often proposed as components of solar cells and other opticoelectronic devices. (In Nature, this type of exchange is called "Matters Arising." )
The comment, "Hey guys, you've got missing planes: Deng, YH. Perovskite decomposition and missing crystal planes in HRTEM. Nature 594, E6–E7 (2021).
From the text of the comment:
It is noteworthy that only the (22¯4), (224) crystal planes appear, and that the (11¯2), (112) crystal planes are missing in HRTEM characterizations in the original paper1. Figure 1a shows the structure of MAPbI3 and Fig. 1b shows the simulated electron diffraction along the [2¯01] zone axis. Clearly, (11¯2), (112) planes exist in the electron diffraction pattern. Moreover, (11¯2), (112) planes are also present in HRTEM images under low electron dose2, selected-area electron diffraction (SAED)3,4 and X-ray diffraction (XRD)5,6,7 characterizations.
Clearly...
Fig. 1: Ball and stick models, simulated electron diffraction patterns of MAPbI3, PbS and PbI2.

The caption:
Additional text:
...In light of above clarification, the structure in the HRTEM images of the original paper1 is more likely to be PbI2, and the higher-contrast spots are caused by the mass thickness contrast effect13. Owing to lack of the corresponding in situ high-angle annular dark-field scanning transmission electron microscopy (HAADF-STEM) image in the original paper, it is impossible to prove that the higher-contrast spots are PbS quantum dots rather than PbI2 particles. Next, the authors should check the experimental conditions of HRTEM, especially the dose of the electron-beam irradiation. If possible, it would also be better if they compared the particle size and size distribution of colloidal quantum dots and quantum dots in perovskite in the original paper.
The original author's response is here: Ning, Z., Gong, X., Comin, R. et al. Reply to: Perovskite decomposition and missing crystal planes in HRTEM. Nature 594, E8–E9 (2021).
In the accompanying Comment2, Deng asks whether there is enough evidence for PbS being embedded into perovskite, and in particular whether TEM images in the original Letter correspond to PbI2 as opposed to perovskite or PbS. Deng noted a difference in the estimated interplanar angle in his analysis of our published data (Deng finds 57°) compared to the value we report (60°). Deng also points out the absence of a diffraction spot related to the (112) plane of perovskite and suggests possible perovskite degradation.
The coexistence of perovskite and quantum dots is supported in the original Letter1 by optical absorption spectra, static and transient photoluminescence spectra, photoluminescence excitation spectra, X-ray photoelectron spectroscopy (XPS), high-angle annular dark-field imaging scanning transmission electron microscopy (HAADF-STEM) and Rutherford backscattering spectrometry (RBS). The density functional theory (DFT) simulations show that epitaxial alignment is possible and is needed to avoid interfacial traps.
In light of Deng’s questions, we revisited the analysis of the interplanar angle and the missing diffraction spots. Since the fast Fourier transform (FFT) is performed on a small subsection of the image to capture the PbS and perovskite lattices separately, the resulting diffraction pattern is diffused, and this leads to a range in determining the centre of each spot, and consequently to a range in angular estimates. Both pre-2015 and contemporaneous 2015 high-resolution transmission electron microscopy (HRTEM) studies also lacked evidence of the (112) plane reflection3,4,5,6,7,8,9. We agree that there is a possibility of perovskite degradation to PbI2 (ref. 3).
Given this possibility, we sought therefore—in light of advances in transmission electron microscopy (TEM) over the past six years—to investigate the materials using improved TEM equipment (aberration-corrected JEOL GrandARM (ARM300F)). We used the method of sample preparation described in the 2015 Letter1. We reduced the electron dose to 10 e Å−2 (e, electron charge), and see the characteristic Fourier spots attributed to {110} facets of MAPbI3 (Fig. 1a, b, d)...
In other words, "we credit what you say Professor Deng, and we went back and did it all over again with instruments that are more advanced than those we had in 2015..."
Fig. 1: TEM analysis of quantum dots in perovskite.
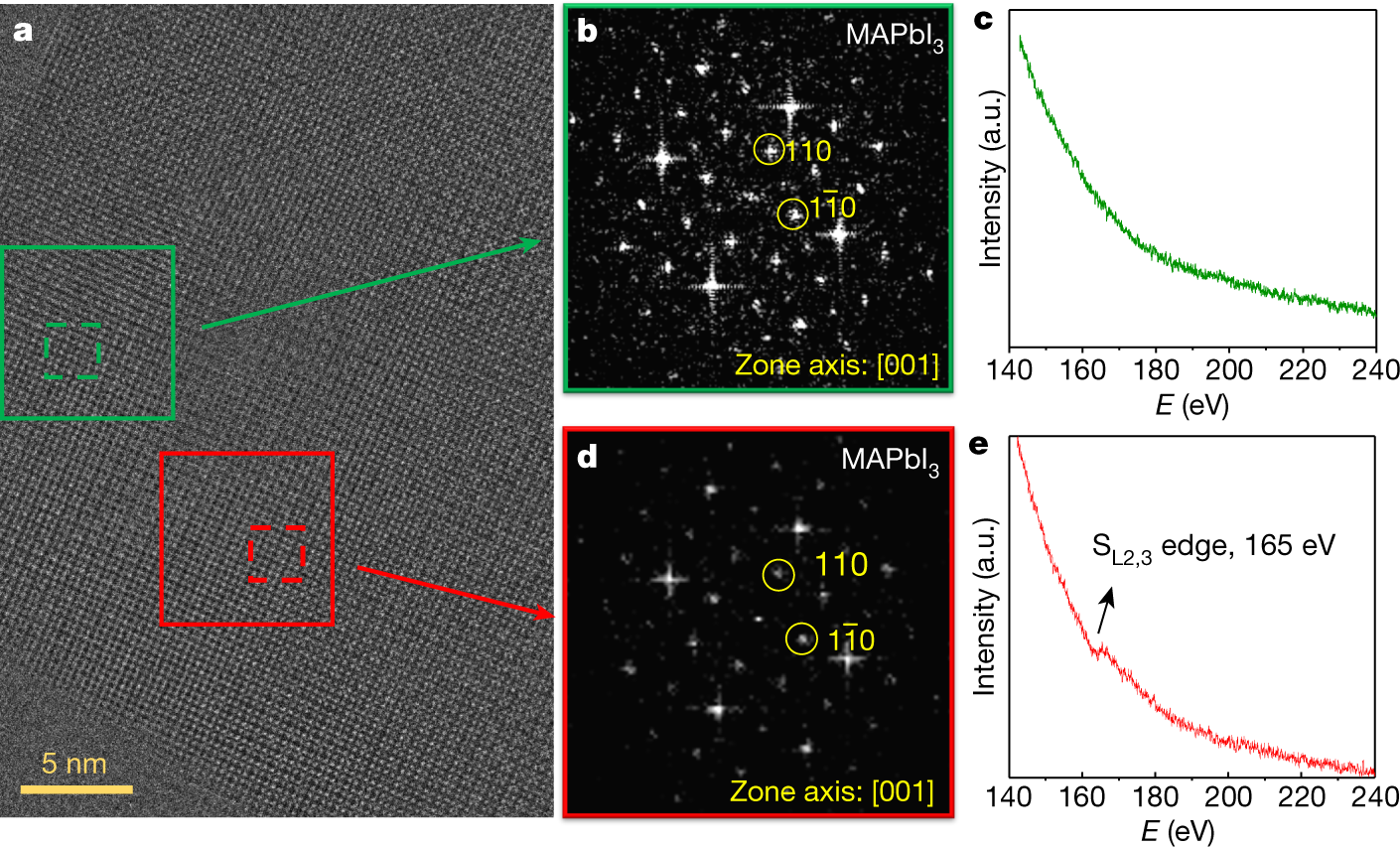
The caption:
Some more text:
The new HRTEM images and the accompanying elemental analysis, together with optical and electronic properties, XPS and RBS from the original Letter, support the finding of dots in perovskite. The evidence of epitaxial alignment comes from the suite of characterization studies reported in the original work. Specifically, DFT reveals that the interfacial energy between PbS (110) and MAPbI3 (110) is less than 10 meV Å−2, suggesting that the epitaxial growth of perovskite on PbS is as energetically feasible as homoepitaxy of PbS on PbS or perovskite on perovskite...
...The use of PbS quantum dots in perovskite has been further investigated by a number of groups. Jung et al. reported using DFT that high-quality heteroepitaxy between PbS (100) and CsPbBr3 (100) was energetically favourable for both materials11. Masi et al. demonstrated the role of lattice matching at the heteroepitaxial interface between perovskite shell (MA) and PbS CQDs and its influence on the optoelectronic properties of PbS CQDs. High mobility (1.3 cm2 V−1 s−1) and high detectivity (2 × 1011 cm Hz1/2 W−1 with >110 kHz bandwidth) was achieved when the lattice constant mismatch is minimized between PbS and the perovskite shell12. Zhang et al. reported, on the basis of HRTEM, epitaxial coherence between the CsPbI3 and PbS CQD lattice13. Liu et al., using TEM, reported that CsPbBrxI3−x perovskites inherit the crystalline orientation of the embedded PbS quantum dots14. Improved performance has been observed in PbS-dot-in-perovskite solar cells, photodetectors, and light emitting diodes13,15,16,17.
We once again thank Deng for having motivated a fruitful dialogue and updates to the TEM studies of the original paper.
A pleasant exchange, I think.
The original paper is here: Ning, Z., Gong, X., Comin, R. et al. Quantum-dot-in-perovskite solids. Nature 523, 324–328 (2015).
I am not, by the way, competent in TEM analysis, and frankly some of this is over my head, but I shared these papers with my son who does a lot of imaging work in connection with his research.
As for lead perovskite solar cells, from my perspective, they are even worse than the existing solar industry since distributed lead solar cells is an even worse idea than distributed solar energy, and distributed energy is a bad idea since it ultimately results in distributed (and thus difficult to ameliorate) pollution. (There's going to be hell to pay for future generations for this pixilated affair.)
It doesn't matter of lead perovskites can make marginally more efficient solar cells. The "solar will save us fantasy" after half a century of wild cheering has not worked, is not working and won't work to displace dangerous fossil fuels and address climate change.
The the science involved with researching the matter, however, is surely of value. Many perovskite structures involve cesium chemistry, and I personally love the fascinating chemistry of that sometimes obscure element.
Have a nice day tomorrow.
The "nuclear will save us fantasy" after half a century of wild cheering has not worked, is not working and won't work to displace dangerous fossil fuels and address climate change.
Have a nice night!
OAITW r.2.0
(31,731 posts)You believe nuclear energy is the ultimate silver bullet solution for humanity's energy needs.
But-
That tech requires big $ from a few people. Labor-less energy is the goal. Risk is Chernobyl / Fukushima Daiichi.
Why not labor intensive wind and solar? Not nearly as efficient, but does make people employed and independent of oil? I will trade 50,000 nuclear, 250,000 oil sector jobs for 1,000,000 solar/wind jobs.
Eko
(9,858 posts)But Nnadir doesn't accept that. For them its one solution to a problem that will more than likely take many solutions. And if you dont agree you are for the killing of a multitude of people every year from fossil fuels from pollution. Ill take reality any day over dogmatism.
OAITW r.2.0
(31,731 posts)turbines are producing.....it's a long ridgeline and I see maybe a 3rd of the entire system. 25 miles away..
NNadir
(37,528 posts)Last edited Thu Jun 10, 2021, 12:32 PM - Edit history (1)
...generations.
Coal mining makes jobs.
Because the solar and wind industry rely on intensive mass requirements, redundant systems, and because these systems have a short lifetime, roughly 20-25 years, they are not sustainable, and they represent an ill considered attack on all future generations.
The uranium and thorium already mined are sufficient in breed and burn scenarios to provide all the world's energy needs for many generations, and each nuclear plant represents a gift to future generations as opposed to the liability that so called "renewable energy" will represent as babies born today enter adult life.
It would be a sane and moral world if people cared as much about the 7 million people who will die this year from dangerous fossil fuel and biomass combustion waste as they do about the bogeyman at Chernobyl and Fukushima. That works out to around 18,000 to 19,000 people per day, more people per day than Covid killed on its worst day.
We do not, of course, live in a sane and moral world, which is why we have these kinds of representations.
Eko
(9,858 posts)Sometimes even closing after getting their 20-year license renewals. https://saplnh.org/about-nuclear/nuclear-plant-lifespans/
Uranium mining leaves behind toxic wastes,
Regardless of how uranium is extracted from rock, the processes leave behind radioactive waste. For example, the solid radioactive wastes that are left over from the milling processes are called tailings and the liquid wastes are called raffinates. Mill tailings and raffinates are stored in specially designed ponds called impoundments. The tailings remain radioactive and contain hazardous chemicals from the recovery process.https://www.epa.gov/radtown/radioactive-waste-uranium-mining-and-milling
The same for Thorium.
Environmental contamination with thorium around RE mining and processing sites is an issue of concern, and many incidences of health impact have been reported. Thorium is enriched during different processing steps like flotation or leaching activities. Therefore, thorium-containing waste arising during RE processing needs a careful waste management since contamination, e.g. by dust drift or tailing dam bursts are severe threats for exposed organisms in the environment
https://link.springer.com/article/10.1007/s40831-016-0083-3
Of course the mining for solar and wind create waste as well, I just wanted to be clear on things.
As for the last paragraph its a straw man that they consistently use so there is no need to address it. I have repeatedly said that I am not against Nuclear and that I think it will take Nuclear and Renewable energy to get us out of this terrible predicament but Im just one of those who think Nuclear is a bogeyman to some. Ill just leave with this again,
The "nuclear will save us fantasy" after half a century of wild cheering has not worked, is not working and won't work to displace dangerous fossil fuels and address climate change.
Have a great night!
cstanleytech
(28,305 posts)By that I mean is what performance level would they need to achieve assuming by some miracle they can to make it viable?
NNadir
(37,528 posts)...is addressing climate change, making for a safe environment, a sustainable world where we can even consider addressing human development goals - for example poverty - the answer is "never."
Continuous processes are always, 100% of the time (where possible), environmentally and economically superior to batch or discontinuous processes.
The sun goes down every day, by most accounts. Thus a solar facility can never be continuous, the very dangerous and dishonest enthusiasm for batteries notwithstanding.
Now there is this caveat, which has only occurred to me in the last few months about photoelectric devices. Many of the perovskite optoelectronic devices contain cesium.
Cesium is a fission product, and in the minds of people who have difficulty thinking clearly or, at least creatively, is "dangerous."
In nuclear fission, pretty much irrespective of the parent nuclide running a reactor, whether thorium/U-233, U-235 (most existing reactors) or Pu-239/241 (my favorite approach) or even americium or neptunium fueled reactors, three cesium isotopes dominate. They are Cs-133 (the natural non-radioactive isotope), Cs-135 (half-life about 3 million years) and cesium-137 (half-life about 30 years.) In a thermal reactor, which represents almost all of the world's existing reactors, the mass ratio of these three isotopes is roughly 2:1:2, because the precursor to Cs-135 is Xe-135, which has the highest neutron capture cross section of all known isotopes.
To my mind, for many reasons, most connected with the very serious but largely ignored issue of chemical pollution associated with persistent organic compounds, most of which are fluorides and chlorides, although many bromide flame retardants are also a serious risk, Cs-137 is the most valuable isotope of cesium, since it's decay product, Ba-135m (half-life about 2 or 3 minutes) is a powerful gamma emitter. Gamma radiation breaks chemical bonds, including very strong chemical bonds, like carbon-fluorine bonds.
Unfortunately, because of the Bateman equilibrium, we cannot produce vast amounts of Cs-137, since ultimately, we will reach a point at which it is decaying as fast as it is formed. Even worse, much of it has been allowed to decay isolated from the environment because of fear and ignorance. Whereas cesium in fresh used nuclear fuel is about 40% Cs-137, in 30 year old fuel it is only about 25%.
Now cesium iodide (natural cesium, nearly pure Cs-133) is a well known and widely used scintillator. That is, when it is exposed to high energy radiation (short wavelength UV, x-rays, and gamma rays) it emits light by a process known as "down conversion." Both cesium and iodide are fission products.
Suppose, for example, we made cesium lead perovskites with radioactive cesium and layered them with radioactive cesium iodide. The result would be a continuous light flux, 24/7, 365.25 days a year, until much or most of the radioactivity decayed to non-radioactive isotopes. Suppose in addition, we surrounded this layered material with a thermoelectric material similar (or better than) the RTG's we send into space. This would represent a new kind of nuclear "battery" inasmuch as it would produce energy useful for any purpose, continuously.
The high energy density of uranium means that this can only represent a marginal, but highly portable and highly reliable form of energy. We might design it so that it is free standing in air and with thin enough layering that a significant portion of the high energy radiation penetrates the (isolated from the public) device, so that the radiation blows apart CFCs, HFCs, SF6, and N2O, all of which are greenhouse gases, and in some cases, ozone depletion chemicals (residual CFCs and N2O).
Now actually I have better ideas than this about what to do with radiocesium than I have time to state here, including ways to "recharge" its radioactivity, but if the goal is to make photocells that are actually useful and reliable, this is one approach that I believe would work quite nicely.
Aside from that, the photocell industry as a tool to produce energy is a rising tragedy.
