Science
Related: About this forumHigh ZT thermoelectric material by doping SnTe with copper and selenium.
Last edited Tue Feb 1, 2022, 08:57 AM - Edit history (1)
A thermoelectric device is a device that converts heat directly into electricity with no moving parts. These devices are best known for the space missions they power, where they are known as radioisotope thermoelectric generators (RTG). From 1961 to the present, they have been utilized on 23 space craft including the famous Voyager, Cassini, New Horizons, and most recently the Curiosity Rover on Mars. The heat source is the radioactive decay of plutonium-238.
The thermal efficiency of an RTG is given by a formula utilizing a dimensionless parameter called "ZT" (it is not a product of two variables) according to the following equation:
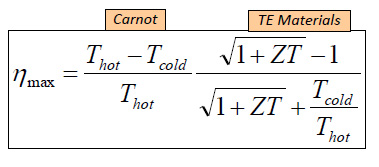
ZT is determined by the following formula:
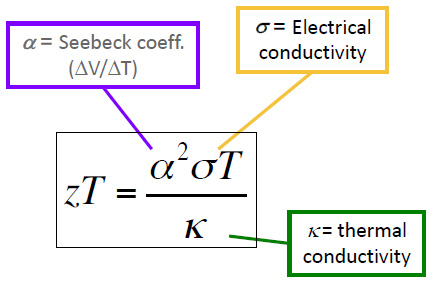
where S, σ, T, and κ are the Seebeck coefficient, (a property of the material) electrical conductivity, absolute temperature, and thermal conductivity, respectively. Note that the value of ZT requires that two variables which normally trend together, electrical conductivity and thermal conductivity - metals usually possess high values of both - must trend in opposite directions to maximize ZT.
The "State of the Art" RTG utilized on Curiosity is the MMRTG (Multimission RTG).
The value of the MMRTG is shown in the following graphic from a 2019 slide show given by Dr. Giacomo Cerretti, a post doc at JPL. The following is also from his slide show, showing where the MMRTG lies in terms of ZT and efficiency.
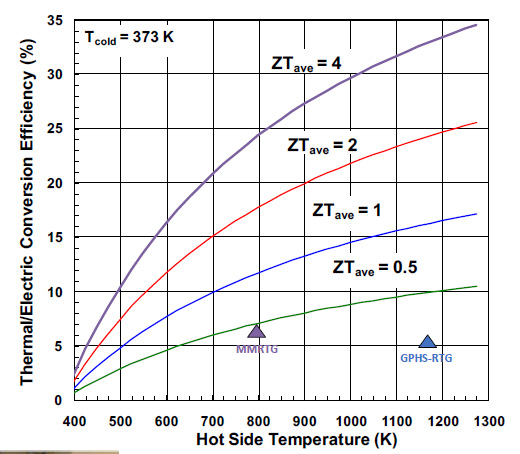
The MMRTG's thermoelectric material is lead telluride.
The thermal efficiency is rather poor, but the device is known to be reliable, hence it's selection for critical missions.
Lead, of course, is a heavy and toxic element. It would be preferable to use a lighter element, and in fact, many space missions have utilized silicon germanium thermoelectrics, although lead telluride is more efficient.
It is known that tin telluride and tin selenide can function as thermoelectrics, but they are not quite as efficient.
It was thus with pleasure that I came across the following paper this evening: Ultralow Lattice Thermal Conductivity and Enhanced Mechanical Properties of Cu and Sb Co-Doped SnTe Thermoelectric Material with a Complex Microstructure Evolution (Samuel Kimani Kihoi, U. Sandhya Shenoy, Joseph Ngugi Kahiu, Hyunji Kim, D. Krishna Bhat, and Ho Seong Lee ACS Sustainable Chemistry & Engineering 2022 10 (4), 1367-1372)
Some text from the introduction:
...Notable state-of-the-art thermoelectric materials include Cu2Se, (4) GeTe, (5,6) higher manganese silicides, (7) Bi2Te3, (8) and SnSe, (9) among others. In addition, tin telluride (SnTe) is a promising group IV chalcogenide which is environmentally friendly and is considerably being sought as a potential replacement for toxic PbTe. (10−12) This is primarily because of the similarity in the crystal structure and tunable valence band structure of both materials. However, SnTe is considered an inferior thermoelectric material compared to its homologue PbTe. This is due to the large number of Sn vacancies that contribute to a high hole carrier concentration, low Seebeck coefficient, small band gap making it prone to bipolar effects at high temperatures, large energy offset between the L valence band and low-lying Σ valence band lowering the valley degeneracy, and high thermal conductivity at room temperature resulting in an overall low ZT...
The authors have chosen to explore improving the properties of SnTe by doping it with copper and selenium.
The results of their efforts can be seen in the following graphic:
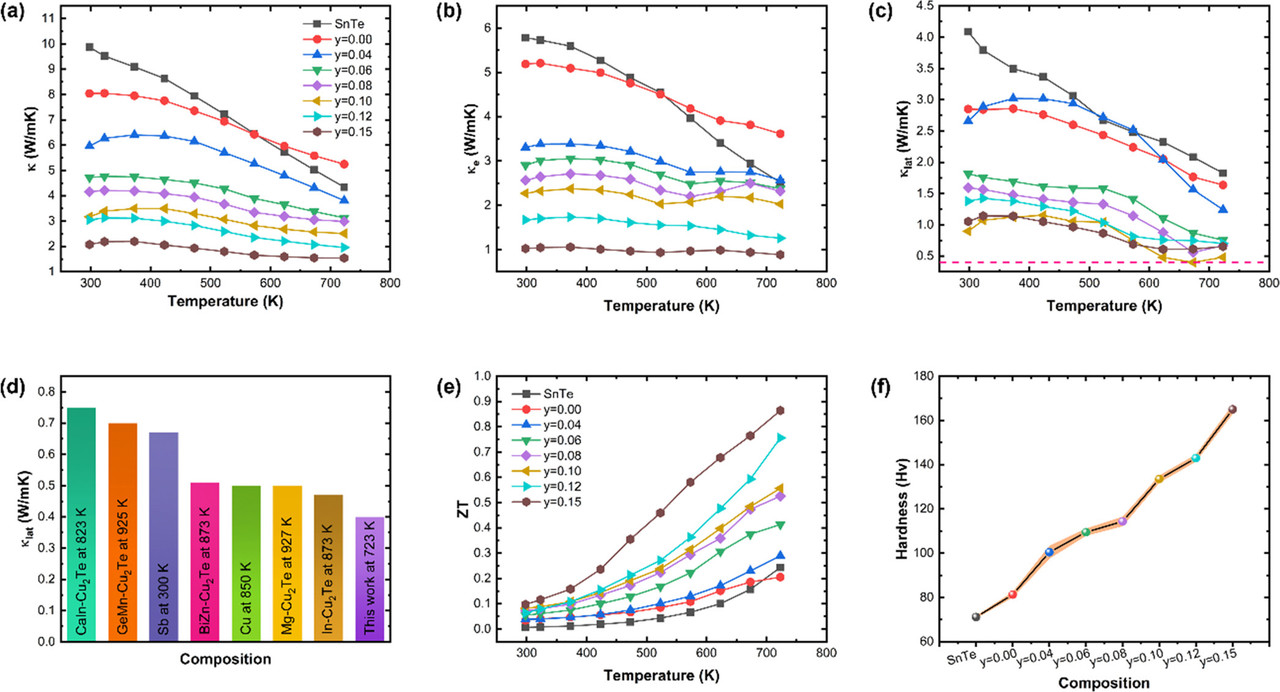
The caption:
It's rather nice, the material in particular with y = 0.15.
It's not clear that this material can be made reliably, but it's nice to see. Thermoelectric devices can be utilized on Earth to capture waste heat. I favor very high temperature nuclear reactors to address climate change because of their potential to obtain high thermal efficiencies via the use of heat exchanger networks to conduct chemical work as well as power generation.
Cool, I think.
EarnestPutz
(2,843 posts)....a couple of the simpler aspects that come to mind. I’ve always wondered about waste heat and why more efforts don’t seem to put into better it’s better utilization. Certainly every time one sees an oil refinery “flaring” waste gas or steam rising from a cooling tower one wonders why this couldn’t be done better. I’m going to guess that these thermoelectric materials work best a higher temperatures than these situations provide, but wonder what you thoughts are on waste heat in general. Also, does your preference for very high temperature reactors mean continuing with very large facilities, such that the various proposals for small scale “neighborhood” or city scale reactors are not feasible?
NNadir
(37,550 posts)Let's start here: The most common use for waste heat, when it's used rather than dumped, is for district heating, which has been around for a very long time, as noted in this recent article in Power Magazine, a trade journal. District Heating from Nuclear Power Plants In general this practice has been in use for dangerous fossil fuel plants; that said, I'm aware that Soviet era nuclear plants, as well as the wonderful Cernavodă heavy water reactors in Romania used nuclear waste heat in this way. If I recall correctly - and I may not - nuclear plants in the old Czechoslovakia did as well.
There is no practical engineering or safety reason why this type of utilization could not be done elsewhere, other than anti-nuke fear and ignorance, which is a palpable cause of climate change in my view.
However, this is probably not the best utilization for heat because the heat has been artificially reduced to low grade heat in current designs.
It would be far superior to utilize the higher temperatures potentially available from nuclear fuels. Modern solid phase fuels in PWR and BWR reactors have a practical limit of around 1500 K (roughly 1200 C) because of material constraints. Other more advanced fuel forms may not have this limit. I personally would like to see temperatures near the temperature at which cerium dioxide decomposes to oxygen gas and dicerium trioxide (cerous oxide). This is around 1400 - 1500 C, let's say 1800 K. This is very high grade heat, even after use, and it can be utilized in a network of heat exchangers to do chemical work (water or carbon dioxide splitting) concrete manufacture and a broad host of other chemical reactions, while still exiting as high grade heat. One can couple two heat engine cycles in various places, Brayton cycles and/or Rankine cycles. Rankine (steam) cycles use water as a working fluid; but there is no constraint for the use of water. A great deal has been written about organic fluids, often HFC's although others have been discussed, to utilize in low grade heat thermal cycles.
These kinds of systems exist - coupled cycles - in dangerous natural gas powered plants, where they are known as "combined cycle plants." The ability to build these plants is tied to the discovery of ways to bond thermal barrier coatings to superalloys in such a way that the gases, which are at higher temperatures than the melting points of the superalloys are, can expand against the gas turbines without melting them. A gas turbine is a Brayton cycle device. The heat exiting the gas turbine is high grade heat which is used to drive a Rankine (steam) cycle. (To my knowledge organic Rankine cycles are not yet commercial.) Modern combined cycle plants have very high efficiencies, approaching, even exceeding, 60%.
I have done crude - very crude - calculations for nuclear plants that might approach 80% thermal efficiency, although some of the products will not be electricity but rather will be chemical fuels.
There are many variants. The limit is economics. The second law of thermodynamics says that no system can be 100% efficient. Each cycle will degrade heat, and one can approach a limit where the extra exergy recovered is not worth the cost of the system.
This, by the way, is the problem with flaring. I am not a petroleum chemical engineer, but I have conducted a large number of distillations in my career. In low pressure distillations it is common for a liquid to become superheated, and "bump," that is to suddenly explode into a gas with high pressure. I would imagine, but do not know, that this situation takes place in a refinery from time to time, with the pressure surges moderated by check valves releasing gases. In theory one could recover these - before I knew better I asked the same question you did, but the cost of equipment to capture these gases, both environmental and economic may not justify the cost. Every system features embodied energy - the energy required to build it - and it may not justify the return for flaring incidence rates. (I know there are efforts to minimize these.)
These costs of embodied energy are the main reason after land intensity that so called "renewable energy" is not sustainable, not clean, and not "green." The so called "renewable energy" industry requires two systems to do what one can do, making neither economically viable and making neither be environmentally sustainable.
As for plant sizing, Rankine heat engines can be of any size. There are certain restrictions on Brayton cycles, since the efficiency is tied through various thermodynamic analyses to pressure differences as well as thermal differences. The efficiency is tied to the compression work, analyzed in the ideal case by isentropic compression, and in the real case, by thermodynamic functions known as a "compressibility factor." These plants thus have optimal sizes in many practical settings.
To return to flaring, the best way to eliminate flaring is to eliminate the use of dangerous fossil fuels completely. The only path to the elimination of dangerous fossil fuels goes through nuclear energy. Wind and solar will not cut it, and their only practical result is to entrench, rather than eliminate, the use of dangerous fossil fuels, as we are seeing in that coal dependent hellhole Germany this winter.
I hope you find this explanation useful.
EarnestPutz
(2,843 posts).....about further, but that's one of the reasons that I enjoy your posts. Thanks again.
eppur_se_muova
(41,327 posts)This is well outside my area of expertise, but I'm kind of fascinated by the seemingly endless stream of intermetallic and metal/semimetal compounds that solid-state scientists are studying, and their diverse and useful properties.
