Science
Related: About this forumRemarkably enhanced direct dissolution of plutonium oxide in task-specific ionic liquid
Plutonium oxide is a challenging substance. The purification of plutonium involves (in the commonly used PUREX process) precipitation of the oxalate and thermal treatment giving the oxide. The oxide can sometimes take a polymeric which is challenging to dissolve.
Therefore I read with interest this paper, Remarkably enhanced direct dissolution of plutonium oxide in task-specific ionic liquid: insights from electrochemical and theoretical investigations Jayachandran, Kavitha , Gupta, Ruma , Chandrakumar, K. R. S., Goswami, Dibakar, Noronha, D. M., Paul, Sumana , Kannan, S. 2019, 1474-1477 Chemical Communications 55 10 2019, 1474-1477.
I don't necessarily agree that the description of electrochemical pyroprocessing in the introductory text but here it is:
In recent years, room temperature ionic liquids (RTILs) have been considered for several applications including the separation of lanthanides and actinides.11 This has been primarily attributed to the unique physical properties of the ionic liquids and more importantly, their less corrosive nature and lower operating temperatures. The solubility, coordination and speciation of f-block elements in ionic liquids have been recently explored.12–15 In spite of the usage of ionic liquids for the separation of f-block elements, the primary issue of poor solubility of lanthanides and actinides in ILs is still unsolved...
The authors explored the use of betaine bis trifluoromethylsulfonylimide (Tf2N-). Betaine is a quaternary permethylated derivative of the simplest amino acid, glycine. (It is a constituent of sugar beets.)
The authors showed the reversible redox capability between Pu(III) and Pu(IV) after showing that his ionic liquid dissolves the hitherto difficult to dissolve Pu(IV)O2. More interestingly the were able to reduce the plutonium to a metallic form on an iron electrode:
Fig 3:
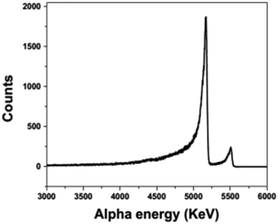
The caption:
The authors continue:
The deposition of plutonium on a steel electrode is something I find very interesting. Plutonium forms a low melting eutectic with iron, and briefly, in the 1960's a reactor operated at Los Alamos using this liquid metal as a fuel, the LAMPRE experiment. While the technology has basically been more or less forgotten, I personally believe it warrants another look for various reasons.
The authors add an interesting note to their paper suggesting a wider utility for this system, noting the work of Chinese workers:
The utilization of plutonium in my opinion is a key to saving what can be saved as climate change engulfs the world as it is doing now (and not in some far off future), and perhaps restore what can be restored of that already lost.
It's a cool, if esoteric little paper.
eppur_se_muova
(41,324 posts)NNadir
(37,547 posts)It's this one: Highly Efficient and Selective Dissolution Separation of Fission Products by an Ionic Liquid [Hbet][Tf2N]: A New Approach to Spent Nuclear Fuel Recycling Fang-Li Fan, Zhi Qin, Shi-Wei Cao, Cun-Min Tan, Qing-Gang Huang, De-Sheng Chen, Jie-Ru Wang, Xiao-Jie Yin, Chao Xu, and Xiao-Gui Feng Inorganic Chemistry 2019 58 (1), 603-609.
They give a nice prep of the ionic liquid which is a simple metathesis reaction, they mix the lithium salt of the imide and the chloride salt of betaine, and the ionic liquid oils out, can be washed with water and made chloride free. I am always interested ionic liquids that are insoluble in water, so I'm making a mental note of this one.
One difference between the extraction used in the paper cited in the OP and the procedure utilized in this paper, besides the temperature (400K, 127°C) as noted by the authors of the Chem. Commun. paper, is that in this case, the ionic liquid is saturated with water.
The authors of the Inorg. Chem. paper just cited have a nice table of solubility for the Ln and An species:
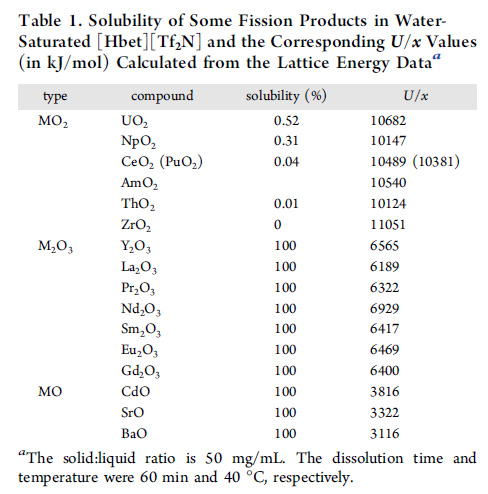
Under these conditions, the water saturated ionic liquid at 40°C, uranium and neptunium oxides are only sparingly soluble, americium not at all, but all of the lanthanides are very soluble. It would be interesting to see how the solubility changes with desiccation by heating and with heat itself. It suggests a path toward continuous crystallization if both papers are accurate, which conceivably as the authors of the Chem. Commun. paper suggest.
I should check out some of the other references in the Chem. Commun. paper, particularly references 17-21, the majority of which are Binnemanns' papers. Binnemanns is a "go to" guy for ionic liquids and actinides.
Betaine, of course, is very cheap and readily available. I don't know much about it's radiation stability, but it's a simple enough molecule that it should be easy to remove any radiolysis products. I know I have a bunch of papers in my files from James Wishart at BNL on the radiation stability of ionic liquids, but I haven't looked at them in a number of years. I'm sure he must have commented on the radiation stability of Tf2N anions. Probably the degradation products are simply triflamides.
These are both nice papers. I like them a lot.
eppur_se_muova
(41,324 posts)The crucible makers chose well.
Looks like there's more than one option to tweak for separation processes there -- variable temp and water content. These guys sound like they're well ahead of the pack and have a good chance of finding a practical way to separate and burn-up actinides.
Some of those other data are unexpectedly interesting to me, as well. Somebody else will probably get to exploit them before I can ever make use of them, but still interesting.
