Science
Related: About this forumAbundances of Some Heavy Elements Result From Fission of Transuranium R-process Actinides.
The paper I'll discuss in this post is this one: Roederer, Ian U., Vassh, Nicole, Holmbeck, Erika M., Mumpower, Matthew R., Surman, Rebecca, Cowan, John J., Beers, Timothy C., Ezzeddine, Rana, Frebel, Anna, Hansen, Terese T., Placco, Vinicius M., Sakari, Charli M., Element abundance patterns in stars indicate fission of nuclei heavier than uranium 2023 Science 382, 6675, 1177-1180.
The heaviest element to survive on Earth is uranium, element 92, but it is understood that the early Earth probably had some plutonium as well, the long lived 244Pu isotope (t1/2 = 81,100,000 years) as well as its decay product, 240Pu with which it must have been in secular equilibrium. (Detection of a few atoms of naturally occurring Pu has been claimed in very old lanthanide ores in California.) A significant fraction of the thorium on Earth, 232Th probably originated as 244Pu.
Speaking of California, the spectrum of the element named for the State, Californium, element 98, has been detected in stars.
Cf. Gopka, V.F., Yushchenko, A.V., Yushchenko, V.A. et al. Identification of absorption lines of short half-life actinides in the spectrum of Przybylski’s star (HD 101065). Kinemat. Phys. Celest. Bodies 24, 89–98
Anyway, the "r-process" is process that takes place during extreme conditions in stellar evolution, think supernovae, neutron star collapses, etc.
The most stable nuclide in the universe is an isotope of iron, 56Fe; lighter elements can release energy by fusing up to iron, heavy elements can release energy (in theory but seldom in practice) by decaying to it. Nuclides heavier than iron, which includes the bulk of the periodic table, although hardly the mass of the universe, which is essentially slightly impure hydrogen and helium, are formed in two ways, both of which are endothermic, i.e. by consuming energy. The first is the s-process, (slow process) in which elements absorb a neutron or proton and then undergo beta or positron decay. This process can take place in stars for billions of years. The second is the r-process, in which very short metastable neutron rich elements continue to absorb neutrons. These processes can result in superheavy elements, and indeed the well known transuranium actinides now available for use on Earth, neptunium, plutonium, americium, and curium, the first three now available on ton scales if isolated from used nuclear fuels.
Anyway, all of the transuranium actinides have significant fission cross sections across all ranges of neutron energies, and it stands to reason that they would be subject to nuclear fission, either by neutrons or by spontaneous fission.
For most elements subject to fission, the distribution of fission products is asymmetric. There's a "light" fraction, centered roughly around the mass number of 90, the stable nuclide at 90 being 90Zr and a heavy fraction, centered around 137, for which the stable nuclide is 137Ba. (These are the figures for the fission of 235U; other nuclides may vary, particularly on the heavy fraction.) The heavier fraction has significant amounts of the lanthanide elements. The lighter fraction has significant amounts of the noble metals, ruthenium, rhodium, and palladium, as well as cadmium and silver. The paper cited at the outset suggests that these elements, the lanthanides and noble metals and just beyond, vary in their stellar abundance because they are the products of fission events in stars.
From the paper:
The detailed compositions of some ancient stars in the Milky Way have been determined from their spectra, which contain hundreds of absorption features of more than 40 r-process elements (9). The abundance patterns of lanthanide elements in these stars are nearly identical, indicating a possible universality of r-process events and producing the same abundance ratios. The composition of each star is dominated by the ejecta of individual r-process events (10, 11), such as neutron star mergers or rare types of supernova, which enriched the gas from which the stars formed (12)...
...We investigated the r-process using a sample of 42 stars in the Milky Way. We selected stars that were previously observed to have heavy elements known to be formed by the r-process, with no evidence of contamination from other processes [such as the slow neutron capture process (s-process)]...
... The heavy-element abundance patterns in the selected stars are shown in Fig. 1 (individual elements are shown in figs. S3 and S4). We found that stars with higher [Eu/Fe] ratios have abundances of some elements (including Ru, Rh, Pd, Ag, Gd, Tb, Dy, and Yb) that are slightly enhanced relative to those in stars with lower [Eu/Fe] ratios. This excess is not an expected consequence of r-process universality...
Figure 1:
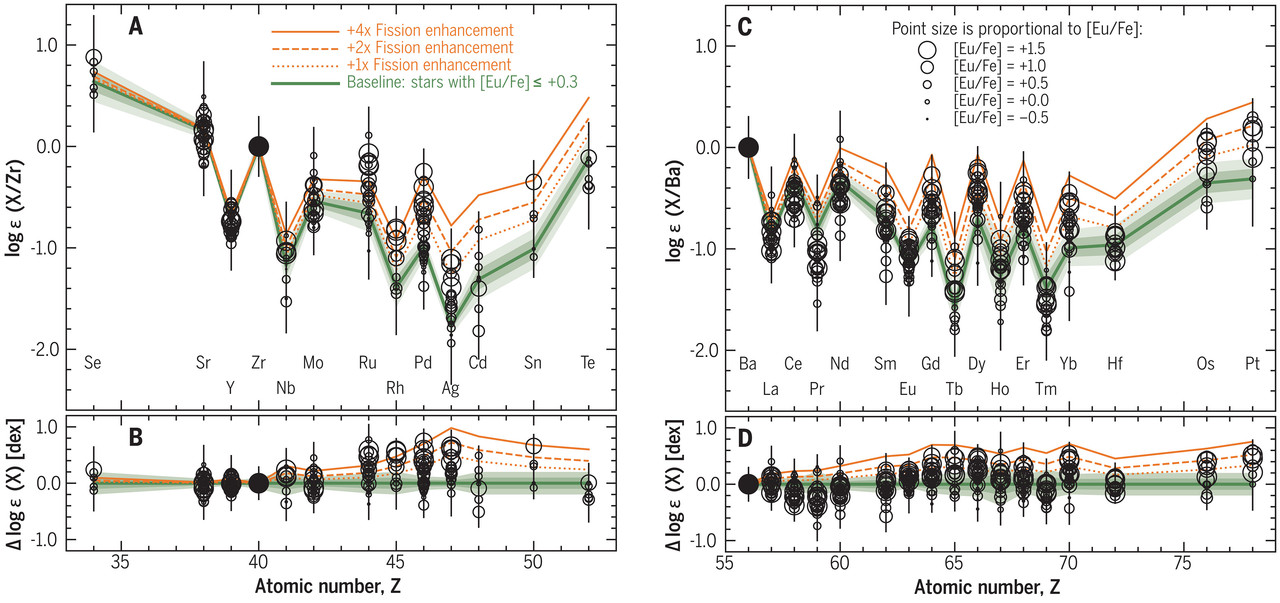
The caption:
Shown are logarithmic abundances (open circles) measured for 30 r-process elements in the 42 stars of our sample, plotted as a function of atomic number. The symbol sizes are proportional to [Eu/Fe], and error bars indicate 1σ uncertainties. The green line is the empirical baseline pattern we defined as the mean abundance ratios for the subset of 13 stars with [Eu/Fe] ≤ +0.3. Light shading and dark green shading indicate ± 1 and ± 2 times the standard error in the baseline, respectively. The orange lines indicate models of fission fragments added to the baseline pattern; the dotted line has equal contributions from the baseline and the fission model, the dashed line has two parts fission fragments plus one part baseline pattern, and the solid line has four parts fission plus one part baseline. (A) Elements 34 < Z < 52, normalized to Zr (solid circle). Elements are labeled at bottom. (B) Residuals between the data and the baseline pattern in (A). (C and D) Same as (A) and (B), respectively, but for elements 56 < Z < 78, normalized to Ba (solid circle). Numerical values are provided in data S1.
This issue has some relevance on Earth, by the way. Some of the fission products in used nuclear fuel are very valuable elements which may be utilized with short cooling periods to eliminate their radioactivity or to make residual radioactivity be of acceptably low risk in use. In particular, the very valuable and technologically important element rhodium is probably more abundant in used nuclear fuels than it is in all of the ores on Earth.
The case for rhodium is probably the exception rather than the rule for valuable elements. While used nuclear fuel contains for instance, several commercially important lanthanides, in particular, lanthanum, cerium, praseodymium, & neodymium that are essentially nonradioactive after short cooling periods, the high energy to mass ratio of the actinides which makes nuclear power environmentally superior to all other energy sources, means that only small fractions of annual demand for these elements can be isolated from used nuclear fuels.
Have a nice afternoon and evening.
