Science
Related: About this forumFRET in biochemistry, not guitars.
It's a good night, as I have learned of a widely used technique of which I have never before heard: FRET: Fӧrster Resonance Energy Transfer, a technique whereby one fluorescent molecule is stimulated in such a way that it causes another to fluoresce, the intensity of the second fluorescence varying with the sixth power of the distance. A means to measure protein-protein interactions.
I came across it in my general reading here: FRET Materials for Biosensing and Bioimaging Ruifang Su, Laura Francés-Soriano, P. Iyanu Diriwari, Muhammad Munir, Lucie Haye, Thomas J. Sørensen, Sebastián A. Díaz, Igor L. Medintz, and Niko Hildebrandt Chemical Reviews 2025 125 (19), 9429-9551.
From the introductory paragraph:
Because FRET can specifically and sensitively measure concentrations, distances, conformations, and kinetics in biomolecular binding and interactions, it is also widely used in biological and biochemical research in situ, in vitro, and in vivo, e.g., in DNA sequencing, structural bioanalysis, enzyme activity studies, or with intracellular fluorescent protein (FP) biosensors. Nanotechnology is another field in which FRET is frequently applied to analyze interactions of nanomaterials and the combination of biotechnology and nanotechnology (e.g., attachment and function of biomolecules on nanosurfaces) is yet another playground for FRET. Apart from sensing, FRET is also used for energy (or exciton) transport in photosynthesis, solar cells, light-emitting diodes, or photonic wires, the design of molecular logic gates, or the creation of physical unclonable functions. Independent of the keyword (e.g., “FRET” or “resonance energy transfer”), the category (e.g., “title”, “abstract”, or “topic”), or the database (e.g., PubMed, Web of Science, Google Scholar), one can find that FRET rapidly developed from a specialty subject at the end of the 1990s to a well-developed research field at the beginning of the 2010s within only ca. 15 years. For more than 10 years, FRET has constantly and strongly continued to populate the scientific literature with approximately four papers published on average per day that carry “FRET” or “resonance energy transfer” in their title or abstract (when performing a standard PubMed search). Many of the FRET application examples mentioned above are discussed in this review and specific references for both review and original research articles are provided throughout the various sections of this review. Over the years, the field has produced many FRET reviews (1−14) and books (15−18) that provide an excellent overview. Moreover, dedicated FRET conferences, (19) a FRET community, (20) and the many FRET research groups around the globe evidence the versatility and importance of FRET.
One thing that makes FRET particularly interesting and versatile is the almost infinite choice of donor and acceptor materials. FPs, organic dyes, or semiconductor quantum dots (QDs) are among the most prominent donors and acceptors but only make up the tip of the iceberg. Gold nanoparticles (AuNPs), which only function as acceptors and nanosurface energy transfer (NSET) rather than FRET should be used to describe the energy transfer mechanism, have also been widely used, especially for in vitro diagnostic applications. While both FRET and NSET abide by the rules of resonance energy transfer, their efficiencies depend on the inverse sixth (for FRET) or fourth (for NSET) power of the donor–acceptor distance. The FRET materials toolbox covers the spectral range from the ultraviolet (UV) to the near-infrared (NIR), excited-state lifetimes from picoseconds to milliseconds, concentrations from single-molecule to millimolar, and bioconjugation methods that provide donor/acceptor attachment strategies to almost any biomaterial imaginable. This unique versatility makes FRET adaptable to almost any environment and applicable for multimaterial and multiplexed biological, chemical, and physical analysis. We previously published a review (in 2006) (21) and a book chapter (in 2013) (22) about FRET materials but the field has been developing so rapidly that many new materials and concepts have been added and optimized over the last 10 years. Whereas this review also covers literature before 2015 and explains basic concepts that have existed for a longer time, we focus our discussion on material developments and applications in biosensing and bioimaging that have emerged over approximately the last 10 years...
This is a review article, and is thus very long. Just two early figures from the text are all I'll have time to post:
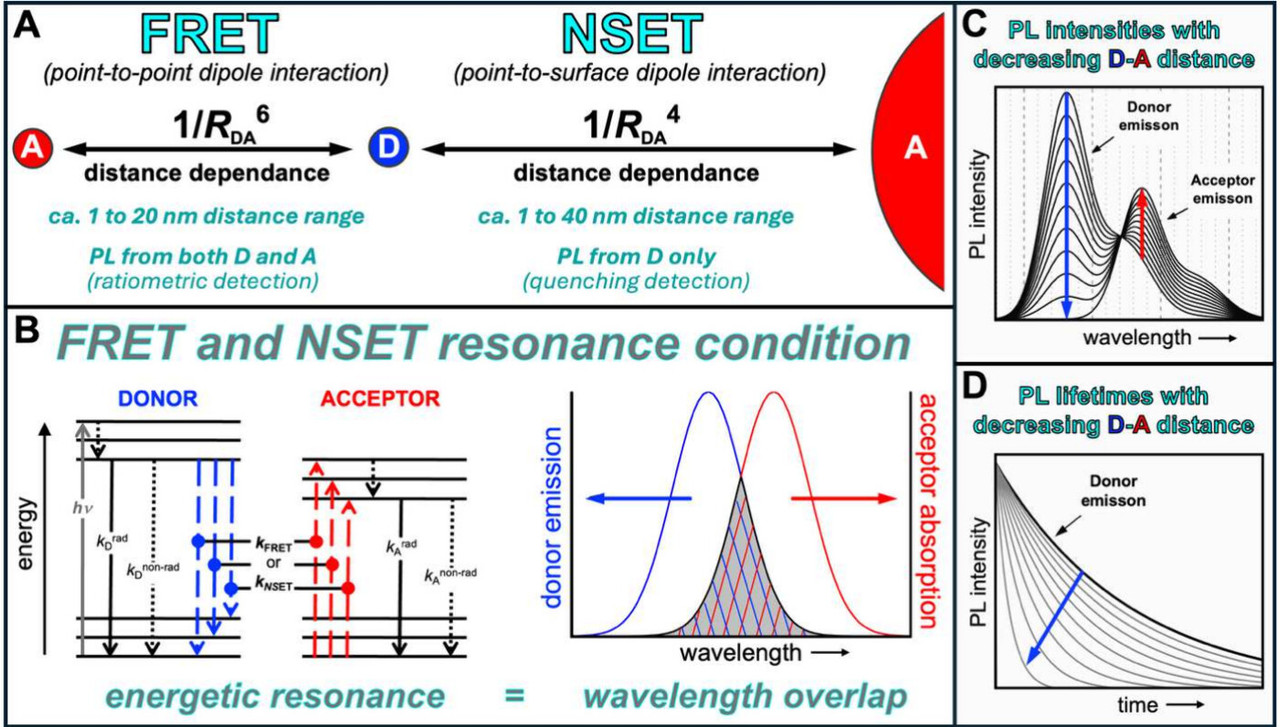
The caption:
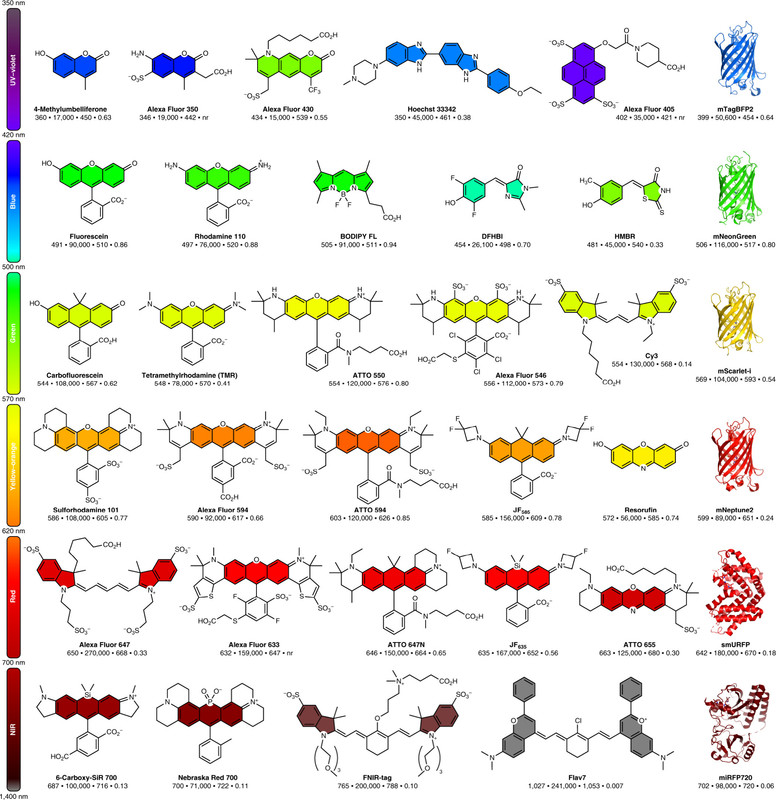
The caption:
The seems like a wonderful way to monitor protein-protein interactions, albeit with a kind of chemical Heisenberg Uncertainty, since the labels potentially would induce steric effects.
Whenever I've been asked about this sort of thing, binding efficiency, being somewhat limited in scope, I always think of mass spec type approaches, HDX and click covalent binding. This technique, despite it's limitations, possible steric interactions, strikes me as superior since it can measure things mass spec can't, specifically distance, conformation, etc, possibly in real time.
Very esoteric, I know, but very, very cool.
I trust the holiday season is rewarding to you, irrespective of your faith or lack thereof.
erronis
(23,127 posts)Thanks for all your stimulating articles this last year. Wisht I could understand 10% of them.
NNadir
(37,553 posts)This year, my wife and I, empty nesters, have a small live Christmas tree, and I'm not messing with its proteome since I'd probably end up killing it.
In the New Year, I wish you many opportunities to wade into things you don't understand, since things like those are the best things in life. That's been my experience anyway.
