Science
Related: About this forumMarie Curie's notebooks are still radioactive and will be for more than a millennium.
This came in on one of my news feeds:
Marie Curie’s notebooks are still radioactive and will be for more than a millennium.
The article is scientifically misleading, since long after 1500 years the notebooks, clothes, etc. will still be radioactive, but after roughly 1600 years they will be half as radioactive as they are now. (The misinterpretation of what a half life is, is actually rather common; when I was a child, I would have made the same mistake as the writer of this article.)
Radium, of course, occurs naturally and is generally, on a planetary scale, in secular equilibrium with uranium. Interestingly, the people who showed that it was possible to induce radioactivity in non-radioactive materials were Irene Joliet-Curie and Frederick Joliet-Curie, respectively Marie Curie's daughter and son-in-law.
The only way to prevent the formation of radium is to fission uranium: It is well known among nuclear scientists and engineers that it is possible, if humanity were to put the transuranium atoms to use, that the use of nuclear power would, after a few centuries, lead to a reduction in the overall radioactivity of the planet, although the planet always has been, and always will be, radioactive.
I noted this elsewhere: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels
The following figure shows the very different case obtained if one separates the uranium, plutonium and minor actinides (neptunium, americium and curium) and fissions them, whereupon the reduction of radioactivity to a level that is actually below that of the original uranium in a little over 300 years:
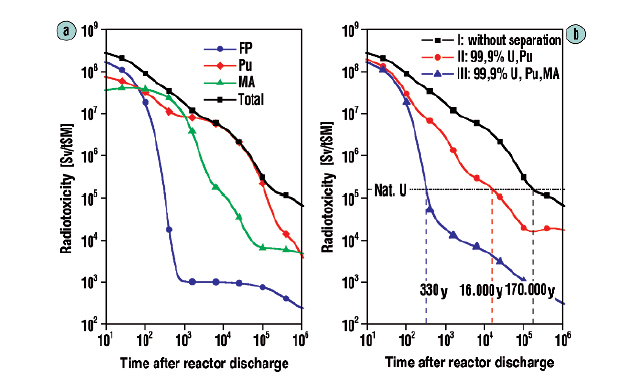
The caption:
(Hartwig Freiesleben, The European Physical Journal Conferences · June 2013)
Source 17, in German, is this one: Reduzierung der Radiotoxizität abgebrannter Kernbrennstoffe durch Abtrennung und Transmutation von Actiniden: Partitioning. Reducing spent nuclear fuel radiotoxicity by actinide separation and transmutation: partitioning.
It is important to note that simply because a material is radioactive does not imply that it is not useful, perhaps even capable of accomplishing tasks that nothing else can do as well or as sustainably. Given the level of chemical pollution of the air, water and land, in fact, the use of radiation, in particular high energy radiation, gamma rays, x-rays, and ultra UV radiation may prove to be more important than ever, but that's a topic for another time...
The article notes that Marie Curie died from aplastic anemia and it is widely assumed, and most probably accurate, that her death was induced as a result of her work in the discovery and industrialization of a radium industry. What is not noted in these accounts is that she lived a relatively long life for her times, dying at the age of 66 in 1934. (She was born in 1867, in Poland.)
Her husband, Pierre Curie, was killed in a road accident. Had he not been killed, it is possible, as was the case of Madame Marie-Anne Paulze Lavoisier, the wife of the "discoverer" of oxygen, Antoine Lavoisier, one of the world's earliest scientific chemists, Madame Marie Skłodowska Curie's work would have been largely attributed to her husband, although they both received the Nobel Prize while he was still alive. (Marie Curie would later receive a second Nobel Prize, making her one of only three people, including Linus Pauling - chemistry and peace - and K. Barry Sharpless, the latter, winner of two prizes in Chemistry, asymmetric synthetic organic chemistry and click chemistry, who is still alive, and who I saw speak a few years back on the subject of how to do science.)
As an aside, the reason that we know as much as we do about the work of Antoine Lavoisier is the work of Marie-Anne Lavoisier, who was a great chemist in her own right. She kept records of her husband's (and her own) work, in which she was a professional as well as personal partner, after his execution by guillotine in the Great Terror of the French revolution.
I had the privilege of seeing Dr. Sharpless speak at the Museum of Science and Industry in Philadelphia, the web page of which includes a short biography of Marie Skłodowska Curie:
Marie Sklodowska Curie
If you find yourself in Philadelphia with some time on your hands, the small free museum is certainly worth a visit. It's near some of the Benjamin Franklin historical sites, which is appropriate, since Franklin was originally famous for his scientific work, before he went on to invent the United States, which lasted well over two centuries before falling because of an ignorant, uneducated, orange pedophile, something of an absurdity, but all the same true.
To return to Dr.Sklodowska Curie
One of the fun things to know about Marie Sklodowska Curie is that after her husband's death, she had an interesting, at the time controversial and scandalous, sex life, famously conducting a fairly well known affair with the married physicist Paul Langevin. Besides being a great scientist, one of the greatest of all time, she was rather attractive physically as a young woman, albeit being, especially in her time - although things are not all that much better in our times - a rare case where her public image generally disregarded her looks in favor of her mind.
Have a nice weekend.
rampartd
(4,476 posts)not only the notebooks, but everthing in and around that lab including her white coat. must have a 1/2 life.
my biggest objection to nuclear power is the disposal of spent fuel rods. somehow burying them for 100,000 years seems a worse idea than melting them down into projectiles for machine guns.
if those elements can be safely recovered i;m all for it.
wolfie001
(7,495 posts)Well-written and the details eye-opening! 💯![]()
evemac
(309 posts)And it is so fortunate that her work was actually attributed to her.
niyad
(131,532 posts)article!
Somebody should tell Marie that we have none-radioactive fairy lights. I have several strings of them.
Old Crank
(6,853 posts)To include which materials and their half lives in the article.
NNadir
(37,800 posts)...below with half-lives included:
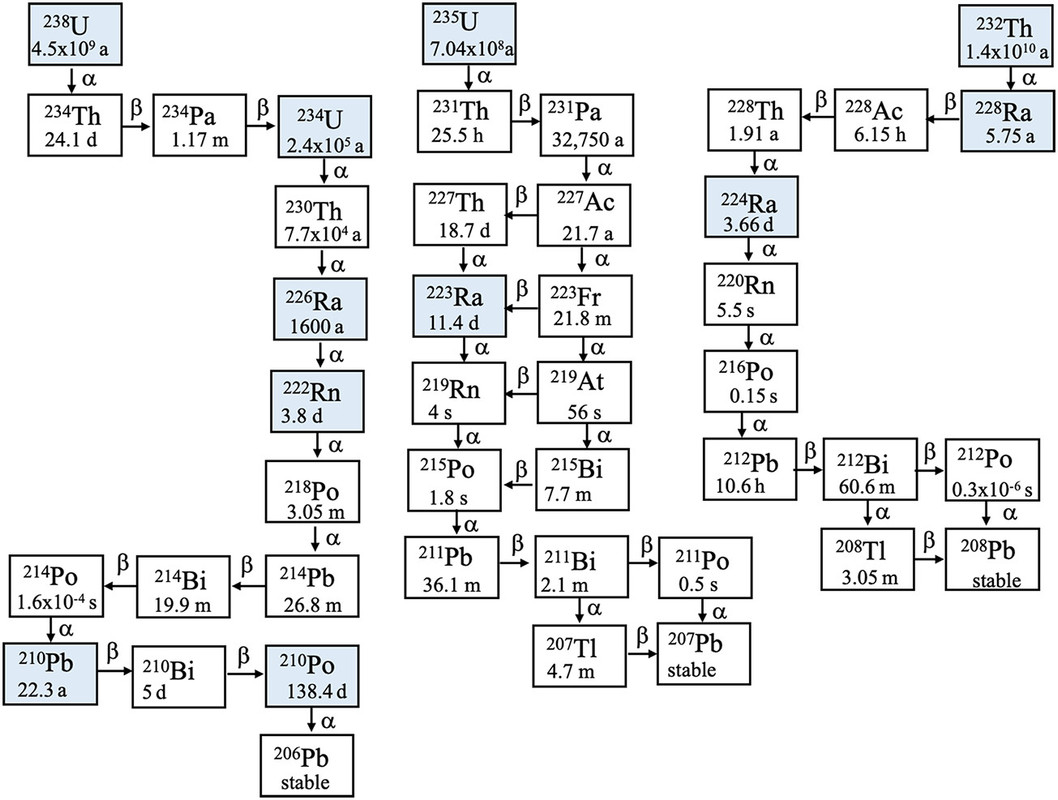
It begins in the chain on the left, 238U at 226Ra, the only isotope of radium that can be isolated in visible amounts. (The half-life in the diagram, 1600 years, is involved in the definition of the nonstandard unit, still in use, the Curie, which is now defined as exactly 3.7 X 1010 nuclear decays per second per gram.
The other two chains shown also occur on Earth, the 235U decay chain, which gives us access to nuclear power, and the 232Th decay chain. There is also a 4th decay chain, that probably once was present on Earth but is now extinct, the 237 Np ( 247Cf) decay chain. This chain has been reestablished on Earth via the use of nuclear power, since 237Np is formed in nuclear reactors, where, when isolated, it has been used to make 238Pu for space missions like the Voyagers, Cassini and Galileo and others. Neptunium can also be used, when enough has been accumulated, as an excellent nuclear fuel where it will be valuable to denature plutonium making it useless for nuclear weapons.
All of the radioactive isotopes below 226Ra are present in Dr. Skłodowska Curie's notebooks and other objects she used during her life, except for 222Rn, which is a gas, representing the largest natural cause of lung cancer, which until people began to smoke tobacco, was a very rare disease. (The parent, uranium, is widely distributed on Earth in vast quantities, notably in most granite, basalt and seawater. It is the major source of heat in the Earth's mantle and inner cores.)
Because 226Ra is a gas, its decay products, shown in the diagram, will be deposited on the walls of the lead containers in which the notebooks and objects are stored. All are highly radioactive with the exception of the stable end nuclide 206Pb. (Much of the lead on Earth started out as uranium.)
All of these decay products have reached "secular equilibrium" which is the point at which the "daughter" nuclei are decaying as fast as they are formed. This situation is also present in so called "nuclear waste," used nuclear fuel; unlike dangerous fossil fuel waste, which, opposed to so called "nuclear waste" actually kills people in vast amounts, fission products cannot accumulate indefinitely. There is a maximum quantity of fission products that can accumulate, depending on the power level of all the nuclear plants operating on Earth, before all of the fission and activation products in them, will decay as fast as they are formed, which can be shown to be asymptotically reached in time. This is why, after a few hundred years in the case where the plutonium and the transplutonium elements are put to use as fuel, that nuclear power will reduce the radioactivity of the planet as a whole, which may or may not be a good thing.
The major radioactive decay products present in the notebooks are those with the longest half-lives a radioactive isotope of lead, 210Pb and 210Po, an isotope of polonium. Polonium was also discovered by Dr. Skłodowska Curie, which she named after her home country, Poland. She was not able to isolate it in visible amounts, but detected it via its radioactive decay and its chemistry. 210Po is the only radioisotope that has ever been utilized in the much hyped and feared case of "nuclear terrorism," having killed one person, Alexander Litvinenko, killed at the behest of the terrorist Vladmir Putin, the person who owns the orange pedophile in the White House. Russia is the world supplier of industrial 210Po which has a number of technological uses chiefly in the elimination of static in the manufacture of products like semiconductors, which are increasingly manufactured on a micro or nano scale.
By using 210Po to kill Litvinenko, the terrorist Putin was able to satisfy the requirement for revenge set out by Edgar Allen Poe in the famous story The Cask of Amontillado, that the person who gets his revenge must be known to the subject of the vengeance. In using this method Putin was able to induce fear in anyone who might seek to repeat Litvinenko's actions.
Thanks for your interest. I hope this helps.
Have a nice weekend.
Orrex
(66,934 posts)NoMoreRepugs
(11,958 posts)BWdem4life
(2,985 posts)NNadir
(37,800 posts)I enjoyed his response which was, "Where is Grammar Eisenhower when you need him?"
cstanleytech
(28,387 posts)NNadir
(37,800 posts)...Radioactive Wolves, ironically produced in the same year as the ballyhooed disaster at Fukushima, where 20,000 people were killed by seawater and collapsing buildings and zero (or close to zero) from radiation exposure.
The documentary gives a marvelous overview of the natural environment of the Chernobyl exclusion zone. It's part of the PBS Nature series.
There was, as a result of the Fukushima natural disaster no effort to ban coastal cities, but by contrast a huge worldwide effort to ban nuclear power. The rationality of this outcome escapes me. One can still find people, right here at DU, who I personally regard as poorly educated and/or easily mislead who carry on about Fukushima but don't give a rat's ass about the vast death toll associated with fossil fuels.
The question of opposition to nuclear power is driven by a number of factors, including but not limited to selective attention exacerbated by bad science driving bad policy. Specifically the bad science is driven by rote acceptance of the LNT hypothesis, the linear no threshold hypothesis. This hypothesis was driven by work conducted in the 1930s and 1940s, a time at which the science of molecular biology was unimaginably primitive. It has been argued, prominently by the health physicist Ed Calabrese, that some of the basic work on the LNT may have involved scientific fraud.
Most people don't realize that they would die without being radioactive since potassium is an essential element and it has been mildly radioactive for the entire history of life on Earth. That's just the tip of the melting iceberg of course.
When I consider public attitudes to nuclear energy, I am often struck by the absurdity, until I consider that there is case where in a once powerful large prominent nation in North America has placed its future in the hands of an uneducated, ignorant pedophile painted a the rather ugly shade of orange. That explains a lot to me.
Nuclear energy is not risk free, but it doesn't need to be so to be vastly superior to all other forms of energy in terms of risk. It only needs to be vastly superior to all others, which it is.
We lost about three decades of nuclear development to the ignorance of antinukes including the "I'm not an antinuke" antinukes who stalk this space. Much of the losses resulting from these pernicious whiners is now irretreivable. That said, we have re-entered an era of remarkable nuclear creativity not seen since the 1950s and 1960s. It is clearly in the realm of "too little, too late," but it's all that we have going forward.
Expecting public wisdom is a rather dubious enterprise.
History will not forgive us nor should it.
Thanks for your expression of bemusement. I share it.