Vagal nerve stimulation for rheumatoid arthritis: A disruptive advance in science [View all]
Last edited Thu Jan 15, 2026, 12:16 PM - Edit history (1)
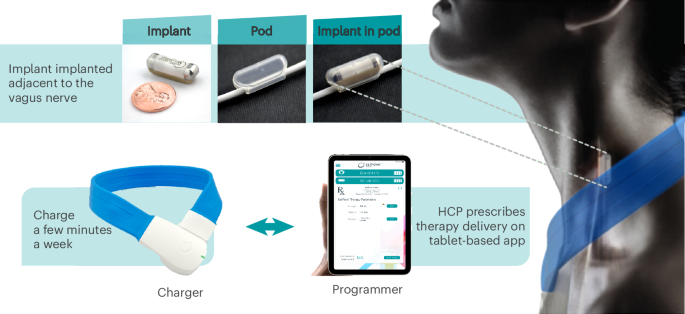
Main
Patients with uncontrolled rheumatoid arthritis (RA) suffer from painful chronic joint inflammation, systemic inflammation and progressive disability due to ongoing structural joint damage. Despite the availability of numerous conventional synthetic disease-modifying antirheumatic drugs (csDMARDs; for example, methotrexate) and biological or targeted synthetic DMARDs (b/tsDMARDs) with distinct mechanisms of action, treatment failure remains a challenge for many patients due to lack of initial response, loss of response over time or intolerance to b/tsDMARDs1,2.
The central nervous system governs homeostatic control of immune responses and bone turnover through innate neuroimmune and osteo-targeted reflexes, including the vagus nerve-mediated ‘inflammatory reflex’. This reflex is dysregulated in RA; tonic vagus nerve activity is diminished, and reduction in vagal tone precedes the onset of clinical disease3,4,5. Actively stimulating the vagus nerve can engage the inflammatory reflex, resulting in acetylcholine release and specific agonism of α7 nicotinic acetylcholine receptors on immune cells6,7. Subsequent modulation of intracellular pathways acting through NFkB, JAK/STAT and the inflammasome leads to reduced production of an array of proinflammatory cytokines (for example, TNF, IL-6, IL-1β

8. This broad-spectrum immunomodulation retains cytokine network bioavailability, thereby allowing reduction and resolution of inflammation while maintaining competent immunosurveillance against foreign pathogens and precancerous cells9,10,11.
snip
Discussion
RESET-RA is the first randomized, sham-controlled trial to demonstrate the safety and efficacy of a neuroimmune modulation device to treat any autoimmune disease, specifically RA. Compliance with therapy and preservation of treatment blinding were achieved through automated nighttime delivery of active stimulation. Efficacy was observed in the 3-month blinded-control period and further supported by improvement during the open-label stimulation period through 12 months, with low usage of adjunctive b/tsDMARDs. In patients with high baseline risk for structural damage, active stimulation substantially reduced progression of bone erosions, as assessed by quantitative MRI joint imaging.
The enrolled study population reflected a spectrum of treatment experiences, including 43% classified with ‘difficult-to-treat’ (D2T) RA, that is, those who had previously failed multiple b/tsDMARDs, with at least 2 different mechanisms of action19, and 39% who had failed a single b/tsDMARD. The patient’s choice to undergo surgical implantation of an experimental device rather than switch to another b/tsDMARD indicated strong patient preference for nonpharmacologic treatment options. Unlike most trials for new therapies in this population, RESET-RA did not impose a requirement for elevated CRP at baseline, allowing inclusion of patients with moderate-to-severe RA regardless of CRP status who would normally be excluded from studies of new RA therapies. This inclusive design reflects the broader United States (US) RA population, where CRP is not uniformly elevated despite active disease20.
]
If I survive Covid I'll be getting one!

