NNadir
NNadir's JournalBiomass Derived Mesoporous Carbon for the Capture of Sulfur Dioxide.
The paper I'll discuss in this post is this one: Facile Preparation of Biomass-Derived Mesoporous Carbons for Highly Efficient and Selective SO2 Capture (Shuguang Deng et al Ind. Eng. Chem. Res. 2019, 58, 14929?14937)
The dangerous fossil fuel and dangerous biomass waste dumped into the atmosphere, familiarly called "air pollution," kills seven million people per year, according to best estimates, suggesting that "air pollution" is too gentle a term to describe the realities of the situation.
Air pollution as a generic term does not in general describe the specific health effects of its constituents, these include particulates, specific carcinogens, generally planar aromatic species like benzofurans, benzodioxins, other polycyclic aromatic hydrocarbons, and inorganic species, particularly carbon particulates, heavy metals including but limited to lead and mercury, as well as acidic gases like nitrogen dioxide, sulfur dioxide, sulfur trioxide, ozone, and of course, possibly the worst of all forms of dangerous fossil fuel waste over the long term, carbon dioxide.
From the general reading I've done over the years, it seems that the component responsible for the most deaths over the short term, other than those resulting from climate change, are the carbon particulates, which may include the polycyclic aromatic hydrocarbons. However none of these gases are trivial in their health effects, and any one of them easily outweigh the health consequences of things popular with small minds, like say, Fukushima, or abandoned uranium mine tailings.
The paper attached here refers to one of the smaller killers among the air pollutants, albeit, again, not at all a trivial killer, since the death toll for air pollution is enormous, sulfur dioxide.
Sulfur dioxide is toxic in its own right, but in air, it is easily oxidized to sulfur trioxide, SO3, which is generally the whitish component of heavily polluted air that one can see pretty much in any major city of the world, particularly on hot days lacking wind. Sulfur trioxide is strictly the anhydride of sulfuric acid, which is a very strong acid, meaning that when exposed to water it forms sulfuric acid, which is obviously toxic and corrosive. It is a component of acid rain, along with nitric acid, which forms from a pollutant also associated with the combustion of dangerous fossil fuels, the nitrogen oxides NO, and NO2 in the presence of oxygen and water.
Sulfur dioxide is produced by the combustion of pretty much any untreated dangerous fossil fuel although there are ways to remove sulfur from the fluid dangerous fossil fuels dangerous natural gas and dangerous petroleum, albeit at expense. If the dangerous fossil fuel is coal which has been the fastest growing source of energy in the 21st century despite whatever lie you may have told yourself (or heard) about coal being dead, the sulfur dioxide (or sulfur trioxide) must be removed after combustion using a scrubber, also at added expense. In the 1970's, scrubbers were added to many coal plants in the United States because many lakes in the Northeast United States were becoming so acidic that nothing could live in them, because of sulfuric acid.
We are now killing bodies of water, including the oceans, with carbonic acids, although to a limited extent sulfur oxides and nitrogen oxides are very much involved in acidification, because we have done nothing effective to address the rising use of dangerous fossil fuels except to prattle on endlessly about how wonderful solar and wind energy are, even though they have proved, after the expenditure of trillions of dollars on them, useless at addressing the rising use of dangerous fossil fuels, acidification of bodies of water, and climate change.
You know the old shibboleth, don't you, sometimes attributed, dubiously, to Albert Einstein? The definition of insanity is doing the same thing over and over again and expecting a different result.
Sulfur dioxide is also produced by the production of many materials, chiefly metals. If one is refining copper, or zinc - the latter also being an ore having the the important industrial elements gallium and indium as side products - the ores are generally sulfides. The metals are produced by roasting them - with heat provided by dangerous fossil fuels - and oxidizing the sulfide to sulfur dioxide which is then dumped in our favorite waste dump, the planetary atmosphere. Steel, including steel made to make all those wonderful massive posts for wind turbines that will be landfill twenty or thirty years from now, also requires significant amounts of coke, which is made by roasting coal while releasing sulfur dioxide both from heating the iron ore and by burning coal to produce the heat.
If one needs to produce redundant systems, say to make copper for a generator for a wind turbine as well as copper for a generator for a dangerous natural gas plant that will run when the wind isn't blowing, one needs to use twice as much as a material as one would otherwise use, meaning that the material derived air pollution will be twice as great as it would if one just built the gas plant, or better yet, if one were actually interested in producing clean energy rather than pretending to produce clean energy, a nuclear plant. (The reason that nuclear energy is cleaner than all other forms of energy is its high energy to mass ratio.)
The world's largest consumer of coal because it is also the world's largest producer of steel, is China. The authors of this paper are Chinese. Nearly half of the papers written in journals published by the American Chemical Society are Chinese. They don't hate science as much as we do.
By the way, one often hears extremely noxious statements that all of our environmental problems are China's fault because China produces more carbon dioxide (marginally) than the United States. This is simply racist nonsense for several reasons: First China makes the metals for our so called "green" stuff, and until recently Chinese workers and children were suffering vast health effects to engage in "green" "recycling" of our electronic and plastic waste, and finally because the average per capita carbon dioxide emissions of Chinese citizens and energy consumption of Chinese citizens is about 1/10th that of average smug American logging on to the Cleantechnia website to read delusional bull about consumerist junk like Tesla electric cars and how hydrogen can be produced from wind and solar energy.
By the way, 99% of the hydrogen produced on this planet is produced from dangerous natural gas. This was true in 1980. It is true in 2019.
In theory, but not in practice, hydrogen can be produced from sulfur dioxide, a topic on which I'll touch briefly here, but right now, it isn't. In terms of the magic words "percent" so widely used when we wish to lie to ourselves about how wonderful so called "renewable energy" is, in 1980 world production of energy from wind, solar, tidal and geothermal energy which generates so much electricity to run computer servers to say how great wind, solar, tidal and geothermal energy are, was close to zero percent of the world energy supply. In 2017, according to the most recent data available in from the international energy agency, IEA 2017 World Energy Outlook, Table 2.2 page 79, these were producing 1.82% of the world's energy. According to this same document, in the year 2000, again in magic "percent talk," dangerous fossil fuels were responsible for 80% of the world's energy supply. In 2017 dangerous fossil fuels were responsible for 81% of the world's energy. The "percent talk" is intended to be misleading and it is. World energy consumption rose by 164.83 exajoules according to the World Energy Outlook from 2000 to 2019, which is the equivalent of adding 166% of a United States to this energy disaster.
So we better know what the hell we're going to do with sulfur dioxide.
From the introduction to the paper written by Chinese scientists:
The Chinese scientists have an idea: They propose to use porous carbon to capture the sulfur dioxide.
...Oil-tea is a unique edible oil and popular functional food in China, and oil-tea shells (OTS) account for ?60% of the camellia fruit on a wet weight basis.16,17 Huge amounts of OTS are produced in southern China annually, and as a lignocellulosic waste, OTS are often discarded directly but without effective utilization. Herein, we prepare OTS-derived porous carbons via a facile one-step activation method. The pore structures could be tuned by altering the porogen/OTS ratio and activation temperature. The as-prepared carbons possess a large surface area, suitable pore size, and abundant basic adsorption sites. An excellent SO2 adsorption capacity of 10.7 mmol g?1 is achieved at 298 K and 1 bar with an outstanding SO2/CO2, SO2/CH4,
A description of their process:
I like the part about the coffee grinder, which gives an idea of the laboratory scale of this work. Often, when we hear breathless stuff about solar and wind breakthroughs - I've been hearing these so called "renewable energy" breakthroughs since I was a young man and I am far from young now, and so called "renewable energy" is still useless - the issue of scale is ignored. All of the massive wind turbines grinding up the avian ecosystem throughout the world, all of the toxic solar stuff that will be electronic waste in 20 years, do not even remotely approach the scale of dangerous fossil fuels, the use of which is surging.
Figure 1 from the paper:

The caption:
Some graphical analytical results of the porous carbon:

The caption:

The caption:
Some graphics about the performance of the porous carbon at removing sulfur dioxide from gas streams produced by the combustion of dangerous fossil fuels:

The caption:

The caption:

The caption:
A cartoon about the mechanism by which the porous oil tea derived carbon works:

The caption:
The paper's conclusion:
This is a fine paper. I like it a lot. Although this work has been performed on a coffee grinder scale, were it go industrial, and the heat for its processes provided by nuclear heat, it would represent something that I think may be necessary for future generations after they clean up our disgusting mess that we generated while cruising mindlessly to the "Cleantechnia" website where we could validate the pretty lies we tell ourselves, and they're not little lies; they're big lies: This technology would sequester carbon by using it.
And let's get real. The economic viability of a process if it is to be sustainable is not to build huge waste dumps, for carbon dioxide or anything else. It is to convert waste into products, to close the mass cycles.
One may ask, though, if the world were to get less stupid and embrace nuclear energy as opposed to endlessly caterwauling with endless selective attention to nuclear energy's imperfections while seven million people die every year because our fantastic embrace of the wait for Godot, the grand renewable energy nirvana that has not come, is not here and will not come, why would we need to capture sulfur dioxide, since we would not be using coal, or oil, or dangerous natural gas.
I want dangerous fossil fuels banned. I think we need to do so on an emergency basis, although it's clear no one cares what I think.
My recent interest in sulfur dioxide stems from one of the conversations I had with my son during our travels back from his internship, a ten hour trip. We were discussing thermochemical cycles.
One of the oldest known and most studied thermochemical cycles is the sulfur-iodine cycle, which involves thermally splitting hydrogen iodide gas into hydrogen and elemental iodine, reacting the resultant iodine after separating it from the hydrogen with sulfur dioxide and water to produce sulfuric acid, and heating the resultant sulfuric acid to its decomposition point to produce oxygen and sulfur dioxide. The net result of this cycle, which is much more thermodynamically efficient than electrolysis, is to split water into oxygen and hydrogen.
I'm not fond of this particular thermochemical cycle, although it is widely studied and is probably the most advanced thermochemical cycle - in terms of understanding and research - of all of them. My concern has been about mass transfer. Iodine has a molecular weight of 254 grams/mole, 258, if one uses slightly radioactive Iodine-129 available from used nuclear fuels. Hydrogen has a molecular weight of 2 grams per mole. I think this is a problem.
But while chatting with my son, a better way of addressing this problem occurred to me. I'm not sure it hasn't occurred to someone else; probably it has; very few of my original ideas prove to be original at all, which I regard as a comforting thing. Anyway, I wanted to share this idea with my son because he is way smarter than I am, will clearly enjoy better opportunities than I have, is developing into a fine young scientist, and I very much encourage him to use his fine mind to save what will be left to save in his times should humanity ever turn away from its increasing investment in, and fondness for, deliberate ignorance.
I will die soon enough, and if I have an idea that may be good, if I share it with him, it may prove useful in a distant time if he steals it, which I encourage him to do.
One of the issues with the oxygen product of the sulfur iodine thermochemical cycle is the separation of two gaseous products, sulfur dioxide from oxygen, and the possibly ersatz "new" idea I had revolved around this separation as well as the reduction of the significance of the mass transfer problem. However, in order to utilize the oxygen produced to capture carbon dioxide from the air, to be environmentally acceptable, the sulfur dioxide must be completely removed from it. Further, if the means to do this is the oxyfuel combustion of biomass or even biomass reforming, biomass contains significant sulfur from methionine and cysteine in proteins and from other constituents in biomass. Sulfur is an essential element for living things, which is why dangerous fossil fuels, which derived from living things, contains it.
Therefore technologies for the removal of sulfur dioxide, including trace sulfur dioxide, are important, and even if this work was intended to mildly reduce the massive noxious results of the use of dangerous fossil fuels, it may have applications in areas intended to arrest, completely, their use.
I hope you'll have a very pleasant weekend.
Trump Aides Go Into Crisis Mode After President's Errant Remarks...
...Condemning White Supremacy.
WASHINGTON—After President Trump openly denounced white supremacy Monday in an errant statement on the mass shootings in Dayton, OH and El Paso, TX, every aide in the West Wing reportedly went into damage-control mode, looking for ways to get him back on message. “Great, now we’re going to be here all night figuring out a way to walk back this offhanded remark,” said White House Chief of Staff Mick Mulvaney, who expressed concern that with a single flippant comment, the president had blemished his administration’s record of consistently siding with and empowering people who believe the United States belongs, first and foremost, to white people. “We’ve worked so hard to make sure he stays on message not only by directly voicing racist views, but by personally embodying bigotry—and now he goes off script and criticizes those exact things! We need to get some dog whistles out to the base immediately.”
Trump Aides Go Into Crisis Mode After President’s Errant Remarks Condemning White Supremacy
I love The Onion.
Key concepts for making informed choices
The linked commentary is from the current issue of Nature and was assembled by an interdisciplinary team of scientists:
Key concepts for making informed choices
I believe this commentary is open sourced, but I will just reproduce the introductory paragraph.
Unfortunately, people often fail to think critically about the trustworthiness of claims, including policymakers who weigh up those made by scientists. Schools do not do enough to prepare young people to think critically1. So many people struggle to assess evidence. As a consequence, they might make poor choices.
To address this deficit, we present here a set of principles for assessing the trustworthiness of claims about what works, and for making informed choices (see ‘Key Concepts for Informed Choices’). We hope that scientists and professionals in all fields will evaluate, use and comment on it. The resources were adapted, drawing on the expertise of two dozen researchers, from a framework developed for health care2 (see ‘Randomized trial’).
Ideally, these concepts should be embedded in education for citizens of all ages. This should be done using learning resources and teaching strategies that have been evaluated and shown to be effective.
We all like to believe that we think critically, but if we are honest with ourselves, we can all recognize instances in which we fail to do so. Given the disruption to the world wide political culture, some of this is worthy, if unlikely to become general practice in the age of twittery.
The rest of the article is at the link, and is, again, I believe, open sourced.
Have a nice day tomorrow.
Molecular Modeling of the Degradation of a Potential Direct Air CO2 Capture Resin.
The paper I'll discuss in this post is this one: Direct Air Capture of CO2 with an Amine Resin: A Molecular Modeling Study of the Deactivation Mechanism by CO2 (Buijs, Ind. Eng. Chem. Res. 2019, 58, 14705?14708)
It is increasingly obvious that we haven't got a practical clue about how to address climate change, since the popular answer, so called "renewable energy" may be soaking up tons of money on a trillion dollar scale, but it has done nothing at all to even slow the increase in the rate of accumulations of the dangerous fossil fuel waste carbon dioxide, the 2nd derivative, and even less to prevent the deaths of seven million people each year from air pollution.
As of 2019, the rate of growth of carbon dioxide concentrations in the planetary atmosphere is hovering around 2.4 ppm/year. In the 20th century, according to the records at the Mauna Loa carbon dioxide observatory operating since the late 1950's, it was (according to monthly data) around 1.3 ppm/year.
Solar. Wind. Blah. Blah. Blah.
Somehow, in the bizarre universe of people at a Trumpian intellectual level for moral and intellectual dishonesty, there are actually people who carry on more about the collapse of a uranium tailings dam in a remote region of New Mexico, which apparently killed no one, with zero attention to the aforementioned annual deaths from air pollution, or for that matter, similar coal mining tailing dam collapses all over the world while we wait for the grand so called "renewable energy" nirvana that did not come, is not here, and will not come.
By the way, about those steel towers for all those wind turbines that will be landfill in 20 years, they're made of steel and to make steel one needs to mine, um, coal.
In the 21st century the fastest growing source of energy on the planet has been coal.
Solar. Wind. Blah. Blah. Blah.
You can actually get people to cheer for this outcome.
As a result of this unconscionable selective attention, future generations are totally screwed; not only will they need to clean up all the toxic electronic waste generated in the useless solar and electric car fantasies, but they will need to clean up the planetary atmosphere that has been greatly degraded because so called "renewable energy" did not work, is not working, and will not work.
For the last 10 years or so, recognizing that there is no political will either on the right or on the left to do what is necessary to address climate change, even to slow it or merely stop its acceleration, represented by a celebration of ignorance throughout the spectrum, I have been considering the very difficult engineering question of how to remove carbon dioxide from the planetary atmosphere, which seems to me to be remotely feasible, but only if we have access to huge amounts of cheap energy, since we need to not only find a way to remove the carbon from the atmosphere, but also to have a place to put it (or better use it), and well as huge amounts of energy to overcome the entropy we have left for people who are infants today.
There are sophisticated arguments in great abundance in the primary scientific literature about this topic, one area of discussion being direct air capture (often abbreviated DAC), of which I am not personally a great fan, although I can certainly imagine some esoteric approaches by which some of this can be practiced, albeit not a tremendously significant scale.
The paper referenced here, which is rather short, examines a case that the author plainly suggests will not work, although the outcome does suggest another possibility for useful organic synthesis using carbon dioxide as a starting material.
From the introductory text:
I am not immediately familiar with the structure of this commercial resin, but I'm guessing that it is an arylalkyl amine of some kind; it is clearly an amine from the context. There may be proprietary issues here.
This is an in silico study:
Here are the graphics from the paper, suggesting the structure of the resin, generally shown edge on, with a few exceptions showing aromatic rings.




B3LYP/6-31G refers to the basis orbital set in the DFT calculations.
Apparently the degradation pathway in this model seems to involve the formation of isocyanates which further react to give ureas.
I personally am always interested in the formation of isocyanates without the use of phosgene, and if nothing else, this paper is of interest in this regard. To the extent that urethanes and polyureas and polycarbonates are synthesized with a carbon dioxide starting material they are effectively sequestered.
This mechanism as described here, in terms of a DAC resin, suggests that this resin will prove as useless for air capture, ultimately generating large amounts of plastic waste, already an intractable problem, much as electronic waste, to which all those shiny solar cells will become in 20 years is an intractable problem.
At least, as opposed to the solar fantasy, this direct air capture will never become sexy and the subject as much wishful thinking as the solar energy scam is proving to be.
From my perspective, direct air capture, as opposed to other more (it seems to me) workable processes seems unlikely to be spectacularly successful, although load leveling by using compressed air, with waste heat being recovered and utilized to minimize energy losses associated with temporary energy storage, said heat available from the output of combined cycle nuclear plants and/or fission products being utilized from used nuclear fuels, may offer some tools available to the generations we have screwed with pure contempt.
Although such processing offers the possibility of addressing and destroying halogenated greenhouse gases, nitrogen oxides and ozone, the effect is likely to be small.
Have a nice day tomorrow.
Molten Salt Synthesis of a MAX Phase Derived Porous Carbon for Supercapacitors.
The paper I'll discuss in this post is this one: Molten Salt Electrosynthesis of Cr2AlC-Derived Porous Carbon for Supercapacitor (Zhongya Pang, Xingli Zou, Xiaolu Xiong, Shujuan Wang, Li Ji, Hsien-Yi Hsu, Guangxin Wu, Qian Xu, and Xionggang Lu, ACS Sustainable Chem. Eng. 2019, 7, 12938?12947)
Once, when I was on a job interview a long time ago where I gave a little lecture to the scientific team there, including the CEO, on the interesting statistics of the random sorting beads into containers, the CEO of the company in my private interview with him afterwards, asked me if I thought I could calculate the fraction of the chemical space he was covering with his combinatorial syntheses. I was pretty young and naive then, so I made a stab at an answer, although I'm not convinced that I really had any real practical idea of how to really approach the problem in a meaningful way. I was offered the job, but declined it. Later I met the CEO of another company who had previously worked in that company and told him I declined a job with it whereupon he remarked that I must be a smart man for not taking the job.
Organic chemistry, essentially the chemistry of carbon, represents a vast chemical space, I would guess essentially an infinite space, and in this sense, a discussion of covering a fraction of it is purely absurd, and a discussion of covering it is, in fact, more than a little arrogant. There are people, of course, who manage manage in magnificent minds huge chemical spaces, but I suppose that even the best minds in the world can keep up with only a minuscule portion of what is there.
Staring into that space, the chemical space of possible organic compounds, is a beautiful thing.
Including molecules like the dangerous fossil fuel waste carbon dioxide and other carbon compounds lacking bonds with hydrogen, the inorganic carbon chemistry seems on the surface simpler. When I was in high school, for example, we were taught that there were two allotropes of carbon, diamond and graphite. Actually a third allotrope was all over the place, and despite the vast efforts of thousands of organic chemists to synthesize it with little success, it was discovered in lampblack, a discovery worthy of a Nobel Prize. This is "buckminsterfullerene" usually referred to in these times simply as "fullerene" a C60 approaching spherical symmetry. It turns out that the chemical space of carbon allotropes is vast. There are not just fullerenes, but also a vast array of compounds like graphene, carbon nanotubes, etc. etc.
When one adds other inorganic atoms, not just, oxygen, nitrogen, and sulfur, but pretty much every long lived element in the periodic table, the inorganic chemistry of carbon is also extremely rich.
An extremely interesting area of inorganic chemistry of carbon, although not strictly an allotrope are a class of materials known as MAX phases, the most famous of which is Ti3SiC2, although many other examples exist. The compounds were first synthesized in the middle of the 20th century, but their properties were basically more or less ignored until the Materials Scientist Michel Barsoum of Drexel University rediscovered them and developed them, a major - and in my view although I don't count - and Nobel Prize worthy body of work. The MAX Phases have the interesting property of offering many of the best properties of metals, high strength, fracture resistance and machinability, the ability to conduct electricity, while offering many of the best properties of ceramics, chemical resistance and high temperature resistance,
One of the interesting properties of the MAX phases is that their structure is extremely regular, as is the case in metals, with the special property that the three elements of which they are composed are highly layered. By exploiting the differing chemistry of the elements within, one can make by leaching one (or two) layers out a class of materials called "MAXenes.
The electrical properties of the MAX phases are different than "normal" metals, and a vast amount of work has been done on the topic. For example, if one enters the search terms (MAX phases anisotropy conductivity) into Google Scholar, limiting the search to papers published since 2015, one will get over 17,000 hits.
A capacitor is a device, as many people know, which consists of two conducting plates separated by a non-conducting substance (a dielectric or electrical insulator). The plates in a capacitor are designed to be charged with opposite charges, and in so doing stores electricity. Although I am extremely rusty when it comes to circuit analysis, I do recall that capacitors are widely used in circuits to filter frequencies out of a circuit; this is known as an "RC circuit," which can be utilized to filter out static signals, extraneous resonances, as well as to manage power surges without wasting all of the energy as a zener diode might do. Also, because the charging of a capacitor can be expressed by a separable first order differential equation the solution of which involves an exponential time constant, properly arranged in circuits, capacitors can be utilized to make oscillators, an obviously important feature of circuit design.
As an aside, capacitors can be utilized in certain kinds of detonators, known as exploding bridgewire detonators that can be made to explode in a very precise manner with precise timing. This makes them important components of nuclear weapons, in particular those depending on implosive shockwaves. To my knowledge, however, no one has ever proposed banning capacitors because they can be used in nuclear war.
Capacitors are widely used in many technologies, most famously cell phones, which can contain hundreds of small light weight capacitors that are based on tantalum. Tantalum, like aluminum, generally contains a passive oxide layer on its surface. This oxide is an extreme dielectric, meaning that layering polished tantalum on top of unpolished tantalum sheets produces a very effective capacitor. This is the most common application for the metal. Tantalum is often considered a "conflict metal" since its mining in the Congo region is often under conditions of essential slavery, often involving small children, an ethical consequence of high technology we'd all rather ignore, just as we ignore the identical situation with respect to cobalt while we worship Elon Musk's car for millionaires and billionaires.
The amount of tantalum in a cell phone is rather small, but given that 1 to 2 billion cell phones are produced each year - there are more cell phones than people on this planet - it adds up.
Depending on the type of MAX phase, as layered structures with a metal, for example, titanium, zirconium, niobium, tantalum, etc, either a semimetal such as silicon, gallium, germanium or the metal aluminum, and a nonmetal (carbon or nitrogen), many types of MAX phases are potential capacitors, at least in the case of being coupled reliably with nanoscale leads. A perfectly formed MAX phase such as the famous Ti3SiC2, as it case of a capacitor, is a layered device with alternating conducting and insulating layers.
Here is the structure of Ti3SiC2:
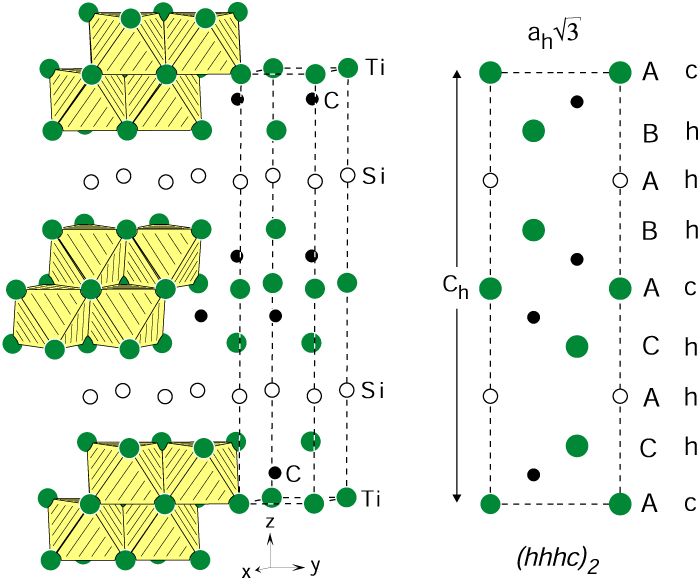
Figure 22: Crystal structure of Ti3SiC2 (space group P63/mmc). The close-packed titanium layers containing the carbon atoms are shown at the left-hand side. The edge-sharing Ti6C octahedra are emphasized. At the right-hand side a (110) cut through the hexagonal cell is shown. The titanium and silicon atoms form close-packed layers with the stacking sequence hhhc, where the silicon atoms correspond to the second h.
Depending on the configuration, in series or in parallel, or a combination thereof, it is possible to make a MAX phase including nanoscale circuitry, into a solid state energy storage device.
Another type of capacitor is the "supercapacitor" which is relevant to the paper mentioned at the outset of this post. Since a capacitor is a device with stable charge separation, it follows that one immersed in a liquid containing ions, or, as in an "ionic liquid" consisting entirely of ions, will, by virtue of charge attraction, also experience charge separation in the fluid. Thus the fluid will, in a sense, be a liquid capacitor as a result, raising the capacitance overall. If the fluid is contained partially within a porous solid phase which is conducting, this solid phase is an electrode, and this charge separation can be utilized to generate a current upon discharge, just like a solid phase capacitor. Under these conditions, one gets "super capacitance," extra bang for the charges in the solid phase.
We generally think of carbon, as a nonmetal as being an insulator. Carbon however has anisotropic conductivity. It conducts when the graphite layers are oriented in one direction, and insulates in the opposite direction. Carbon electrodes are well known and widely utilized, particularly in electrolytic technologies such as that utilized in the aluminum industry, or, in the recently developed FFC Cambridge process which famously can be utilized to produce cheap titanium and many other metals. In a supercapacitor, carbon in various allotropes, nanotubes, graphene, and fullerenes, for example can thus function as an electrode. In order to maximize the containment of ions in the fluid, as well as to allow for large surface areas for discharge, porous carbon is ideal, which is the subject of this paper.
From the introductory text:
Carbide-derived carbon (CDC), prepared by removing metal atoms from carbide precursors, exhibits great potential for the electrode material of supercapacitors because of its excellent properties (such as tunable porosity, structural versatility, and high specific surface area, etc.).17?22 Currently, the primary method utilized for CDC synthesis is chlorination process. Through disposing of the carbide precursors by hot chlorine gas, metal chlorides and derived carbon can be achieved, and the general reaction in this process can be expressed as xMC(s) + (y/2)Cl2(g) ? MxCly(g) + xC(s) (where MC is metal carbide, MxCly is gaseous metal chloride).17,23?25 However, this chlorination process inevitably arouses environmental concerns because of chlorine gas. Besides, the pore structure of the CDC produced by the chlorination process generally only consists of narrow micropores, which commonly restrict the electrolyte accesses into the pore structure.19 Actually, effective electrolyte penetration usually comes from the contribution of mesopores, 26 and thus, it is crucial to create mesopores during the etching process. Besides, the chlorination process commonly needs to be operated at high temperature.27 The pore structure can also be influenced by the carbide precursors.21,22 Therefore, seeking a new green process and appropriate precursors for the synthesis of CDC with controllable micropores/mesopores ratio is still necessary...
What follows is a description of the MAX phase Cr2AlC, a max phase with two metals and a (nominal) nonconductor, as opposed to Ti2SiC3 which has two insulators and one metal.
The structure of these materials will be shown in some of the graphics shown below. What is interesting in this process is that it is a molten salt etching process.
Molten salts are just what they sound like, salts heated to beyond their melting points. The technology of using these is not particularly exotic; they have been industrial use for quite some time, in the Hall process, to which I alluded above for the production of aluminum, and the also mentioned FFC Cambridge process. The production of fluorine gas has long utilized molten salts, a mixture of KF and HF in a ratio of 1 : 2.30 in an electrolytic process; it also works with rubidium and cesium albeit at higher cost. A rather famous nuclear reactor operated in the 1960's, the molten salt reactor (MSR) which utilized a low melting eutectic of BF3 and LiF known as FLIBE. (I used to be very fond of these types of reactors, but I don't think now that they are the best kind of reactors possible; I would hate to be in a position of advocating for the expansion of mining beryllium, a notoriously toxic metal.) The discovery of a wide array of what have come to be known as "ionic liquids" - some exist naturally in living systems, for example choline choride) has vastly expanded, essentially to infinity, the number and types of molten salts that can exist.
In this paper a "traditional" molten salt has been used, one very similar to that used in the FFC Cambridge process.
From the experimental text:
Some graphics and pictures from the text:

The caption:

The caption:

The caption:

The caption:

The caption:

The caption:

The caption:

From the conclusion, summing up what went on in the full paper:
Super capacitors have been the subject of much attention in the popular "wishful thinking" memes that go around endlessly as the atmosphere collapses relating to so called "renewable energy," a trivial and useless form of energy that has not addressed climate change, is not addressing climate change and will not address climate change. All of this attention has been directed toward bourgeois fantasies about storing energy from wind and solar energy which is notoriously unreliable without it (and even more environmentally questionable with it).
Energy storage, whether in a battery or in a capacitor, wastes energy. Capacitors are devices which generate significant heat, one can see pressure release expansion disks on some which are designed to prevent them from exploding, except of course, as mentioned above, in precisely timed detonators. It is one thing to have capacitors in circuits designed to accomplish some task, as in the operation of communication devices and computers, another to storing so called renewable energy. There has never, not once, been significant so called "renewable energy" to store in any case; the combined wind, solar, geothermal and tidal energy output has yet to produce 2% of the nearly 600 exajoules of energy humanity consumes each year. The use of dangerous fossil fuels is accelerating, not coming to an end. Dangerous natural gas is not "transitional;" it is increasing ensconced as an unconscionable burden on all future generations. Representations to the contrary are simply pretty lies we tell ourselves.
This realization is beginning to emerge from the shadows, even among those not enthralled by the international rise of simplistic fascist and racist impulses which while not helping the situation, are hardly uniquely responsible for it.
In any case, this little somewhat meaningless scientific aside is, if nothing else, interesting.
I trust you're enjoying your workweek.
The Obituary of Susan Eaton, from the Members of Her Lab.
A tribute to Susan Eaton, the evolutionary biologist raped and murdered last month in Greece:
PDF version
Suzanne Eaton, who died tragically last month (Nature 571, 305–306; 2019), was our leader, our role model, our mentor and — most importantly — our friend.
Academic research can be arduous and unrewarding. During those dire times, Suzanne met us promptly. She insisted on going through all the raw data with us. By embracing each result as a potential clue to the truth, she taught us how to analyse and think about it in depth. And she could amplify any tentative excitement of our own with an exclamation such as “What? Wow! That’s fascinating”, accompanied by an exuberant banging on the desk.
It was important to Suzanne that the special experience of working as a team was never compromised. She always asked our opinion before offering someone a position.
Suzanne taught us to think synergistically. She showed us links between seemingly unrelated topics. The sources of several discoveries made in the lab could be attributed to such leaps in her thinking. Perhaps this approach is what we need to answer the overarching questions in science. Her illustrious life and career are a testament to that vision (see Obituary Nature Cell Biol., in the press). We shall all do our very best to honour her memory.
Nature 572, 178 (2019)

She was more than a victim. She was a scientist, and a mentor to scientists.
A moving tribute in the age of misogyny and other lies.
July 2019 is the third worst July recorded at the Mauna Loa CO2 observatory in 60 years.
I keep spreadsheets of the monthly and weekly data at the Mauna Loa Carbon dioxide observatory which I can use to sort and interpret the data. The data going back to 1958 is posted on the observatory's website, 61 years of data and 60 years of year to year monthly comparisons. July 2019 was recently posted. July 2019 is the third worst ever recorded.
Monthly Average Mauna Loa CO2 (Accessed August 11, 2019.)
The average reading in July 2019 was 411.77 ppm as compared to 408.71 ppm for July of 2018, 3.06 ppm higher than last year. (The carbon dioxide levels fall every year from roughly May to September during the Northern Hemisphere's summer.)
Of the twelve worst monthly readings, 7 have occurred since January of 2015. The 3.06 recorded this month is the 24th worst of 725 monthly year to year increase readings, placing it the 97th percentile for all such readings.
The worst July, 3.40 ppm over the previous year occurred in 1998, an El Nino year in which fires set to clear the Malaysian and Indonesian rain forests to make palm oil plantations to supply "renewable biodiesel" to Germany and other countries went out of control, burning much of the rain forest in those regions and releasing the carbon.
The average year to year increases for every month since June of 2009 is 2.34 ppm/year. The average for the first ten years of such data from June of 1959 to June of 1969 was 0.82 ppm/year.
If any of this troubles you, don't worry, be happy.
Head over to E&E forum here and read posts where you can be comfortable about how agreeing with this paper, Evaluation of a proposal for reliable low-cost grid power with 100% wind, water, and solar (Proceedings of the National Academy of Science, Vol 114 Iss. 26 6722–6727 (2017)), is a "right wing talking point."
Maybe you'll feel better. I won't, but maybe you will.
Let's not all of us on the left claim we take science seriously.
For half a century, we've all been hearing all this stuff about 100% renewable energy by "such and such a year." "Such and such a year" is in Godot territory; it never comes; it won't come. So called "renewable energy" did not work, is not working, and, I am absolutely certain, will not work to even slow the growth rate of dangerous fossil fuel waste concentrations in the atmosphere, the clean and unambiguous cause of climate change, never mind stop it. In fact, when this rhetoric received world wide support at a level of trillions of dollars in this century, the degradation of the atmosphere accelerated as opposed to slowed.
I'd love to stay and chat, but I'm reading some scientific papers on the absorption of sulfur dioxide into ionic liquids. I had a wonderful chat with my son on this topic on our trip back from ORNL and I hope he'll keep in mind to rescue what might be rescued in the future we've destroyed.
During the trip I informed him that even though my generation has been abysmally selfish, and his generation did not deserve what we have done to them, leaving them with piles of garbage, a lack of resources, and widely distributed and ubiquitous poisons, it is nonetheless the responsibility of people with fine scientific minds like the one he possesses to devote themselves to humanity and not to repeat our awful example, for each such person of high ability (and even low ability) to do what he or she can.
Have a nice Sunday.
Determining the Flux of Anthropomorphic Iodine From Nuclear Power Plant Radioiodide Releases.
The paper I'll discuss in this post is this one: Atmospheric Iodine (127I and 129I) Record in Spruce Tree Rings in the Northeast Qinghai-Tibet Plateau
Only two elements in the 5th period of the periodic table are essential to life, Molybdenum, because of its role in nitrogen fixation enzymes, and iodine.
The overwhelming bulk of fission products produced in nuclear reactors, from natural spontaneous decay of terrestrial uranium, and nuclear weapons tests, are in the 5th period of the periodic table which stretches from rubidium to xenon. In the 4th period only selenium, bromine, and krypton are present in appreciable amounts, and the radioactivity associated with only one of these three elements is of any concern - bromine in used nuclear fuel is not particularly different than natural bromine, and selenium-79 is produced in very low yields; the isotope of "concern" being krypton-85. (In general, "concern" about nuclear materials can border on Trumpian scale stupidity; the loss of life from commercial radioactivity is dwarfed by the loss of life from things nuclear energy can easily replace. In the 6th period, cesium and barium and the first half of the lanthanides (up to around gadolinium) are significant fission products, but again, the bulk of fission products are 5th period elements.
Molybdenum, really a wonderful element, when obtained from used nuclear fuel after a few weeks of cooling is not particularly different than mined molybdenum and although the high energy to mass ratio of nuclear fission means that commercial quantities of this element obtained from used nuclear fuel will never be significant, there is no particular reason that it couldn't be utilized for normal commercial uses.
Natural iodine is mostly the stable I-127 isotope, this isotope being the only stable isotope. Iodine-131, has a short half life, about 8 days, meaning that its release to the environment is generally a serious matter, since it has a high specific activity. This isotope was of significant concern in the Chernobyl and Fukushima events, since iodine is a volatile element which is easily transported in the environment. The high activity of I-131 is also exploited for medical use, ironically most prominently in the treatment of thyroid cancer and certain hyperthyroid conditions.
Iodine also has a relatively long lived isotope, I-129, which has a half-life of 15.3 million years, an isotope is naturally present in the environment from the spontaneous decay of natural uranium (primarily in the oceans), but whose presence in the environment has risen significantly, largely because of the reprocessing of used nuclear fuels in Europe, an activity which, by the way, I enthusiastically support although it is subject to huge processing improvements.
From the cited paper's introduction:
129I, a long-lived radioisotope of iodine (T1/2 = 15.7 Ma), has been released to the environment by human nuclear activities, including nuclear fuel reprocessing plants (NFRPs), nuclear weapons tests (NWTs), and nuclear accidents (NAs). The anthropogenic 129I (NFRPs: 7400 kg; NWTs: 150 kg; NAs: 7.2 kg) has highly overwhelmed the natural inventory in surface environment (250 kg, with a prenuclear 129I/127I atomic ratio of 1.5 × 10^(–12)).(5,6) Due to the unique source of anthropogenic 129I, volatile properties and biophilic characteristics of iodine, the anthropogenic 129I has been widely applied as an environmental tracer for investigation of regional radioactive sources and the related atmospheric transport pathways by determination of 129I preserved in time-serial samples (sediment, ice core, and coral samples).(6?12) However, due to the insufficient resolutions of sediment samples caused by low deposition rates in many locations, the sampling difficulties, and specified locations of ice cores and coral samples, they are not sufficient for the investigation in large areas, especially in midlow latitude terrestrial environments.
Tree ring is an excellent time-serial material to monitor past climate changes as well as anthropogenic activities due to its wide distribution, easy access, annual resolution, accurate chronology, and environmental sensitivity,(13) which might preserve the environmental radioactivity information both in the prenuclear and nuclear age and in different locations. Dendrochemistry based on stable isotopic composition and elements concentrations in annual rings as retrospective proxy data has been successfully used to investigate the past environmental change, such as past temperature and precipitation (?18O, ?13C, etc.),(13) and the anthropogenic release of toxic elements to the atmosphere (Pb, Cd, Hg, etc.)...
From this text we can see that the bulk of I-129 released into the environment, estimated to be about 7.4 tons, comes from the rerprocessin of nuclear fuels.
By the way, this radioactive iodine has more or less been deliberately released to the environment to save money, which in my opinion is probably a good thing, since it very unlikely that spending money to contain it forever would save lives, since it is very unlikely that the release of iodine-129 has killed anyone, but even if I am wrong, and it has killed someone, the ratio of the number of people killed by such releases is dwarfed by the number of lives saved because dangerous fossil fuel waste was not released. It is immoral and stupid to argue that spending millions upon millions of dollars to save one or two lives, whether or any lives lost are subject to irrational fetishes, is worthwhile when the same amount of money spent otherwise could save thousands of lives.
In any case, the anthropomorphic release of I-129, as the authors note, is an excellent tracer.
Later in the introductory text they note:
This work aims to investigate the sources, transport pathways, and transfer of atmospheric iodine in the QTP by determining 127I and 129I in tree rings of spruce. The feasibility of using tree rings of spruce to record the temporal variation of iodine isotopes in the atmosphere will be investigated in order to obtain a historic record of 129I and 127I levels in the atmosphere; this is useful for reconstruction of the levels of radioactive fallout and providing a fundamental database for regional environmental change research.
(Lop Nor is the site of the Chinese nuclear weapons test site; China was one of the last countries to conduct open air nuclear weapons tests.)
The analytical portion of the text is quite interesting inasmuch as it says something about radioactivity and iodine. Because the half-life of I-129 is so long, and thus its specific activity so low, and because the ratio of it to naturally occurring (and essential iodine is so low, parts per ten billions) the I-129 is not detected by its radioactivity but rather by the use of a very sensitive mass spectrometer, the Agilent 8800 ICP/MS. These instruments have sensitivity on the order of parts per trillion, as well as a wide dynamic range, meaning that they can easily pickup the signals of iodine-129 and iodine-127 without too much difficulty.
This demonstrates why the release of I-129 is a trivial matter from a human (and animal) health perspective.
Some pictures from the paper:

The caption:
The total iodine in the tree species in each year represented by a tree ring and the ratio of radioactive iodine to stable iodine:

The caption:
Ratios recorded with events and practices:

The caption:
Comparison with iodine in Greenland:

The caption:
Some comments on those nuclear accidents that raise such a bugaboo, even though the massive death toll from the normal operations of dangerous fossil fuel plants, don't even justify a fart's worth of intelligence:
Chernobyl:
Fukushima:
Now, in my position, as a person who claims that nuclear energy and only nuclear energy is sustainable enough to address climate change and the massive death toll associated with dangerous fossil fuel waste, I am sure that some of what is written above will upset a certain set of people who actually are not even knowledgeable enough about nuclear issues to know how much knowledge they actually lack on the subject, but my purpose is not to address these people, since they are clearly beyond hope.
For the record, I believe there is good reason to recover iodine from used nuclear fuels, assuming that we can get more used nuclear fuel, something which, in my view, as a scientist, is an urgent imperative for humanity to accomplish, which is not to say that humanity is wise enough, collectively to do so. Ignorance is more powerful than it has been at any time in the last 70 years, and the situation is getting worse not better.
By the way, the authors have determined that the flux of anthropomorphic natural iodine has increased significantly in the last half a century, apparently by an order of 3. There's no surprise there. If anything marks our generation, it is our profligate use of the elements in the periodic table, with no regard whatsoever for the needs of future generations.
I wish you a pleasant weekend.
Two years ago, I sent my son an email. He called me up to discuss it tonight.
Two years ago I emailed him to say that he really shouldn't go to his university unless he knew all about the Kirkendall effect.
Apparently he didn't read it until now.
It appears he finished his lab work and written reports and posters on his summer internship at ORNL and was so bored doing nothing he decided to read emails with papers his old man sent him over the last two years.
He told me he has never had to consider the Kirkendall effect, despite my advice that he had to know it to start his university education, but expects he will have to do so soon.
He then wanted to know why I had a strange dark obsession back then with telling him all about the Peng-Robinson equation. I asked him why people he knows insist on using the Beattie-Bridgeman equation instead.
Then we argued about peritectics.
Little brat!
Then he made me jealous by telling me about his visit to the IHFR and the cadmium lined neutron beam tube.
I'm driving him home this weekend, ten hours in the car, not counting breaks. It will be like heaven. I love that boy, I mean, that young man, and it's not only because he's smarter than I will ever be or have been, but for many other reasons, too many to count.
I probably do not deserve all this joy.
He's not my candidate, but Beto's righteous anger thrills me.
"What the fuck!!!!" Indeed.
The media made this monster.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,515