NNadir
NNadir's JournalMy wife says I have to warch Bad Bunny. I don't know who he is.
Neither does she.
(I actually hate football, but we always watch the Superbowl.)
It appears that one of the cats we adopted from a shelter is a pedigree breed.
We adopted two cats some months back, a topic I've discussed in this forum previously, a mother and son. The mother is obese and her son is a juvenile delinquent, but we love him anyway.
My wife was poking around for what to do cat obesity (which we're slowly managing). She came across a picture of a pedigree cat, a British Shorthair, a breed generally gray in color, think body mass, and striking yellow-orange eyes:

British Shorthair Cat: Characteristics and Care.
The picture is almost identical with our cat Kiki, with white paws, a little bit more white around the neck. (Most British shorthairs are pure grey, but many look like Kiki.
(Her son is clearly a mixed breed, but he has his mother's striking eyes.)
We have no idea how Kiki ended up on the streets of Newark, NJ, pregnant with her son, who is now our second cat Harry, our juvenile delinquent who has confused himself with a flying squirrel. She may have escaped from a breeder situation, a kitten mill. We just don't know, but her history, given to us by the shelter made it clear that she was picked up on the streets of Newark before being transferred down to our local shelter, where she lived with her son, in a cage for a few months.
For the record, I'm not into pedigreed pets, and never would have bought one, but we do love Kiki, a very sweet cat with a wonderful personality, which is apparently a feature of the breed, consistent with who she is.
Determining the Source of Water from the Combustion of Dangerous Fossil Fuels by Oxygen Isotopes.
The paper I'll briefly discuss in this post is this one: Measurements of Combustion-Derived Water Vapor Isotopic Composition from Different Fossil Fuels Meng Xing, Junji Cao, Zhoufeng Wang, Qiyuan Wang, Wenwu Cai, Jie Tian, Jianjun Li, and Weiguo Liu, Environmental Science & Technology 2026 60 (3), 2615-2626
Oxygen has three stable isotopes, overwhelmingly dominated by the doubly magic isotope 16O. (Eight is a "magic number" in nuclear physics, isotopes have a magic number of protons or neutrons or both are unusually stable. sup]16O has 8 neutrons and 8 protons. ) The other two stable isotopes are sup]17O and sup]18O, which respectively account for roughly 0.04% and 0.2%, depending on source, as noted in the paper.
I have always wondered how much of the water in the atmosphere comes from the combustion of dangerous fossil fuels, and the paper suggests an answer.
From the paper's introduction:
Generally, the isotopic composition of NWV undergoes fractionation through hydrological and biogeochemical processes, including evaporation, transpiration, atmospheric transport, mixing, biological utilization, and atmospheric chemical reactions. (13) In contrast, CDWV originates from the oxidation of H atoms in fossil fuels with atmospheric molecular oxygen (O2). Consequently, the δ2Hv of the resultant water vapor molecules preserves the original isotopic imprint of fossil fuel-bound hydrogen, while their δ18Ov is jointly governed by the isotopic characteristics of O atoms in both the fuel matrix and atmospheric O2. During biological synthesis, organisms exhibit preferential utilization of lighter hydrogen isotopes (1H over 2H), a process termed biosynthetic discrimination. This metabolic selectivity results in deuterium atom depletion (δ2H values more negative) in fossil fuels relative to the Vienna Standard Mean Ocean Water (VSMOW) reference. (14,15) Consequently, CDWV inherits this 2H-depleted signature. In contrast, the δ18Ov of CDWV predominantly reflects the isotopic inheritance from atmospheric O2, which exhibits a characteristic δ18O value of +23.9‰, (16) and results in its signature being markedly enriched compared to NWV...
The oxygen in the combustion gases partially result from atmospheric oxygen, and the authors discuss this issue extensively.
Several figures in the text show this point, mixing ratios, but I will not have the time to elaborate on this point from the paper.
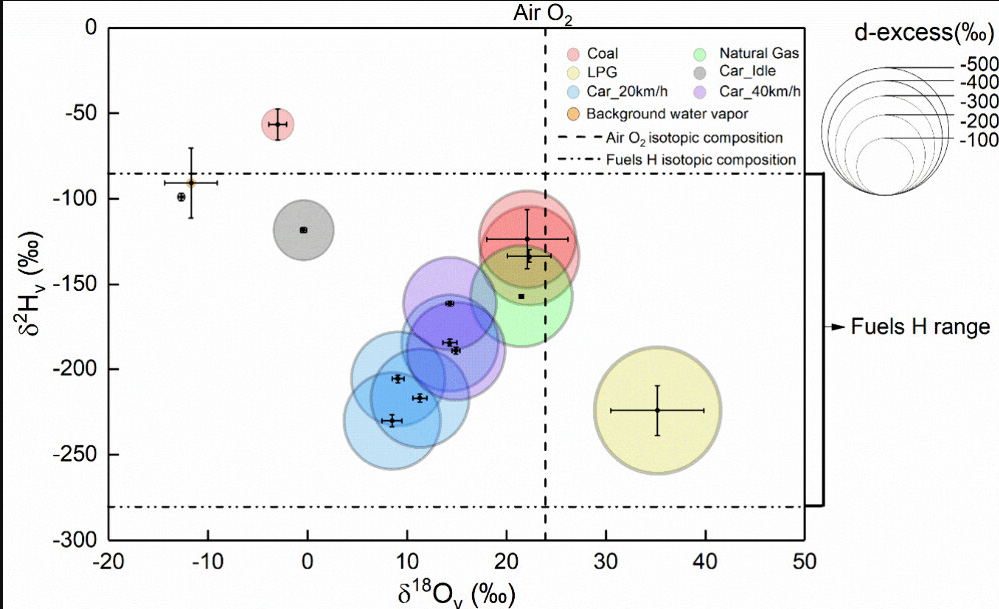
Here, however is one figure from the text:
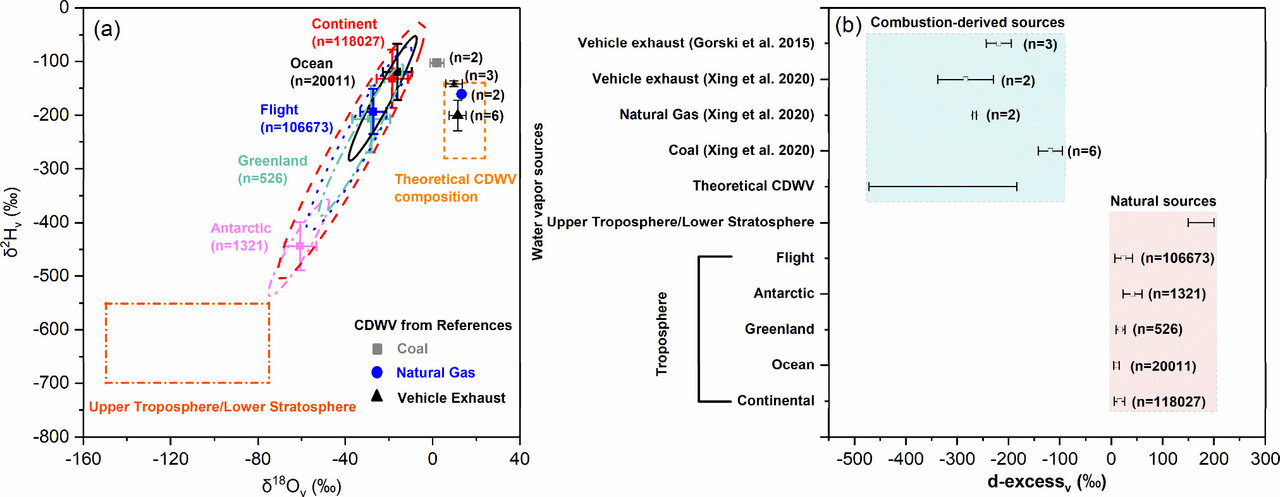
The caption:
Figure 1. Variation ranges of water vapor δ18O and δ2H in the troposphere (data from Wei et al. (18)), upper troposphere/lower stratosphere (data from Galewsky and Samuels-Crow; (19) Sayres et al.; (20) Yang and Yoshimura (11)), theoretical CDWV (data from Gorski et al.; (6) Fiorella et al. (1)), and reported CDWV in references (data from Gorski et al.; (6) Xing et al. (7)) (a); the same as (a) but for the variation ranges of the d-excessv values (b). Error bars represent one standard deviation of the mean, with the sample sizes (n) provided in parentheses. Theoretical CDWV composition and upper troposphere/lower stratosphere data merely indicate the ranges of variation. In panel (b), blue shading indicates combustion-derived water vapor sources, and red shading indicates natural water vapor sources.
Tables from the text:
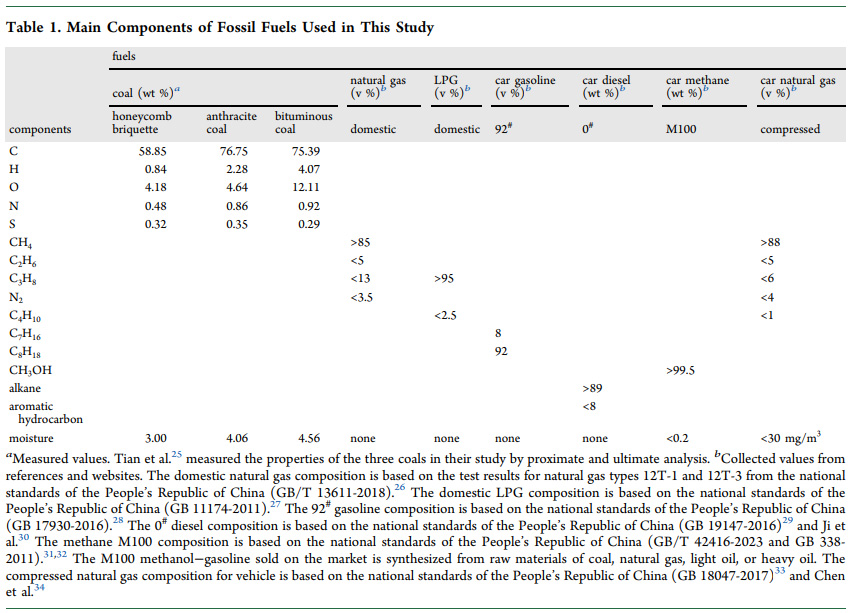
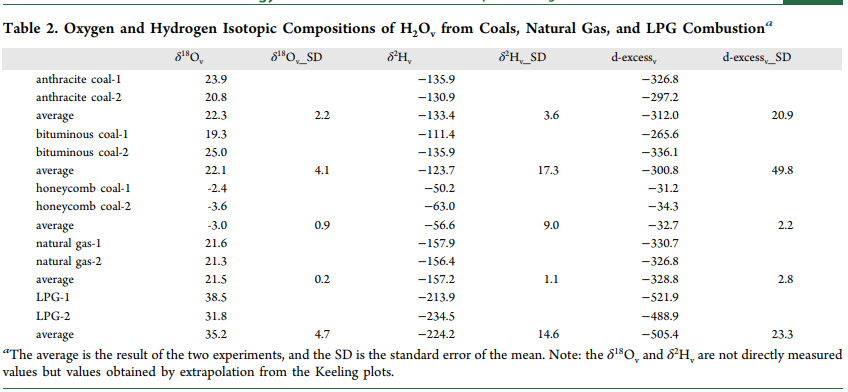
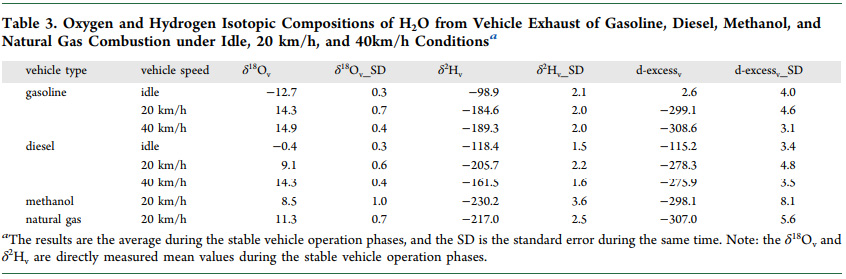
An additional figure from the text:
The caption:
The technique used for measurements is one with which I have limited familiarity although I know that it is used at the Mauna Loa Carbon Dioxide Observatory, Cavity Ring Down Spectroscopy, which is based on the decay of emissions generated by exposure of a gas to laser light.
The authors rightly discuss the limitations of the work and avenues of work building upon it.
Have a pleasant Sunday, even if you are, as I am, in the extreme cold regions of the country.
A Really Fabulous Lecture We Saw Today on the Topic of AI, Computer Vision, Language and Robotics: Deep Learning.
My wife and I went to the Princeton Plasma Physics Laboratory's Ronald E. Hatcher Science On Saturday Lecture Series this morning.
The speaker and her topic was Dr. Kristin Dana, giving a lecture entitled: Training Robots: Deep Learning for Embodied Artificial Intelligence.
Before attending the lecture I thought this one would be the least appealing to me, but, as Dr. Dana is a wonderful speaker and an incredibly aware scientist as well as clearly a great teacher, I think it is one of the best lectures in this series I've seen over many, many years. It will be on line in a few weeks in the archives, and I recommend it.
AI is a very controversial topic here, on which I may be swimming against the DU stream, as I have done for many years with my views on nuclear power, although in that case, the current against me with respect to nuclear issues is clearly slowing, a good thing in the face of a collapsing atmosphere, maybe too little too late and the failure of the antinuke's so called "renewable energy," but well...
Dr. Dana is a very aware thinker, and the audience raised many questions touching on the ethical and sociological issues with AI and robotics. She certainly didn't come across as all "peaches and cream" about the topic. During the Q&A many people raised points about the ethical issues. (I wish I had asked the question I asked differently.)
Others raised the environmental issues, which are very real. The silver lining, for me at least, is that it is driving the development of nuclear power, something I regard as essential to saving the planet. (Nuclear issues were not discussed in this talk.)
I am not opposed to servers and computational power, while recognizing that of course these things are subject to abuse. (I am mildly amused when I read complaints on DU, a server dependent website.)
Like all human technologies it has both positive applications and others which are dangerous, even to the point of frightening. One of the interesting points was that people developing AI often don't understand exactly how it works on a deep level; they are often surprised that its capabilities exceed their expectations.
The question of course is kind of 1950's science "fictioney" to coin a phrase, the question being, will humans make themselves obsolete? After the lecture, I kind of understand the risk on a deeper level.
Again, the talk will be on line in a few weeks. I recommend it highly. It raises questions and makes one think.
I trust you're having a pleasant weekend.
None of My Mom Friends Are Dying
Last night I posted a reference to a memorial included in a scientific journal, to an important environmental scientist, Dr. Katherine T. Peter, who died young, shortly after giving birth to her first child.
The post is in the E&E forum and is here: In Memoriam: The Scientific Legacy of Dr. Katherine T. Peter
Before dying, Dr. Peter wrote a moving piece about being terminally ill, which is also, in my view, valuable inasmuch as it stands as a warning that one should trust one's body as much, perhaps more, than one's doctors, since doctors, even excellent doctors, as human beings, are subject to making mistakes.
Dr. Peter's piece is here: None of My Mom Friends Are Dying
An Excerpt:
None had excruciating pain during pregnancy, unrelenting constipation or unexplained blood in their stool. None went septic five days postpartum or were ultimately diagnosed with stage IV colon cancer with liver, lung and peritoneal metastases. None are parenting a two-year-old, knowing that they might not see him turn three.
It’s not surprising that I’m unique among my friends in this way. Colorectal cancer (CRC) during pregnancy is rare, occurring in one woman per 50,000. Applying this ratio to the 3,664,292 US births in 2021, I’m one of seventy-three extremely unlucky women. Such cases often involve delayed diagnoses, because CRC symptoms often present when the disease is relatively advanced, and are masked by pregnancy symptoms. Looking back at my pain-filled pregnancy, the clues were there—but no one deciphered them until too late.
I was so excited to become pregnant, determined to stay physically active and continue my usual busy routine as an environmental scientist, hiker and quilter. Unfortunately, my actual experience was very different. By mid-second trimester, I was enduring left-sided abdominal cramping that made even a thirty-minute walk challenging. I was also extremely constipated and had blood in my stool, but my obstetrician explained these as common pregnancy symptoms.
At thirty-two weeks and six days, the cramping suddenly became a sharp, agonizing pain, folding me in half. The next morning, at my thirty-three-week appointment, my obstetrician recommended more stool softeners and told me stories about other patients who’d experienced pain during pregnancy. I remember feeling baffled, and guilty about complaining.
Is this awful pain truly typical third-trimester discomfort? I wondered. Do I just need to be tougher?...
It's a moving piece, but also something of a warning.
NJ Legislature Gather at Princeton to Kickoff the 250th Commemorative Session
NJ Legislature Gather at Princeton to Kickoff the 250th Commemorative SessionMembers of the Assembly and the Senate convened at the Faculty Room, shaking hands and catching up before the 250th commemorative General Assembly session started.
Princeton University President Christopher L. Eisgruber welcomes the NJ Legislature to Princeton University to hold a ceremonial session at Nassau Hall.
Assembly Speaker Craig Coughlin, a Woodbridge Democrat, announced the event during his remarks at the reorganization of the 222nd Legislature last month.
“To commemorate the first session, this February we will return to Nassau Hall for a special session as we begin a yearlong celebration throughout our wonderfully historic state,” said Coughlin. “As we remember the past we should reflect on the proud accomplishments over the past several legislatures"...
...Before Nassau Hall became a part of the Ivy League school, the New Jersey Legislature held its first meeting in the building on Aug. 27, 1776. As the nation went to war for independence from England, members gathered to adopt the state constitution and establish a new state government. The historic landmark later served as a military fortress for both sides, the Loyalists and the Patriots, during the war. William Livingston was elected the state’s first governor and delivered the first speech to a joint meeting of the Legislature in the building.
Presiding Thursday, Coughlin gathered ayes and nays after each selected Assembly members’ resolutions about both Princeton University and the Legislature’s histories, as well as the American Revolution’s impact on the state and new nation.
Assemblyman Robert J. Karabinchak, an Edison Democrat, said, “I’m so proud of New Jersey. Being here in the hall where it started and thinking about the history that was here is just amazing…This is where it started and this is where we will continue to do great work for the state.”
Me too, Assemblyman Karabinchak. I am proud of New Jersey, including thinking, of the role it played in establishing the United States, a great country until its fall in recent times.
Marie Curie's notebooks are still radioactive and will be for more than a millennium.
This came in on one of my news feeds:
Marie Curie’s notebooks are still radioactive and will be for more than a millennium.
The article is scientifically misleading, since long after 1500 years the notebooks, clothes, etc. will still be radioactive, but after roughly 1600 years they will be half as radioactive as they are now. (The misinterpretation of what a half life is, is actually rather common; when I was a child, I would have made the same mistake as the writer of this article.)
Radium, of course, occurs naturally and is generally, on a planetary scale, in secular equilibrium with uranium. Interestingly, the people who showed that it was possible to induce radioactivity in non-radioactive materials were Irene Joliet-Curie and Frederick Joliet-Curie, respectively Marie Curie's daughter and son-in-law.
The only way to prevent the formation of radium is to fission uranium: It is well known among nuclear scientists and engineers that it is possible, if humanity were to put the transuranium atoms to use, that the use of nuclear power would, after a few centuries, lead to a reduction in the overall radioactivity of the planet, although the planet always has been, and always will be, radioactive.
I noted this elsewhere: 828 Underground Nuclear Tests, Plutonium Migration in Nevada, Dunning, Kruger, Strawmen, and Tunnels
The following figure shows the very different case obtained if one separates the uranium, plutonium and minor actinides (neptunium, americium and curium) and fissions them, whereupon the reduction of radioactivity to a level that is actually below that of the original uranium in a little over 300 years:
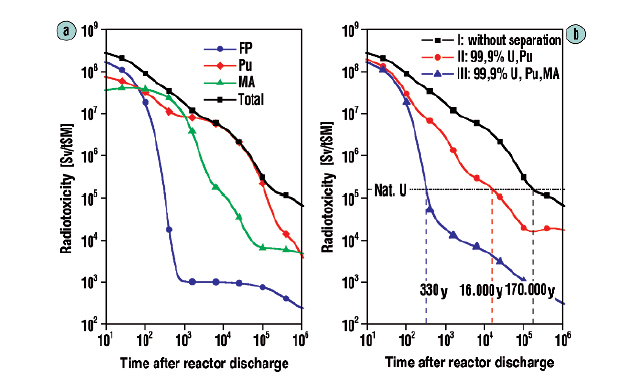
The caption:
(Hartwig Freiesleben, The European Physical Journal Conferences · June 2013)
Source 17, in German, is this one: Reduzierung der Radiotoxizität abgebrannter Kernbrennstoffe durch Abtrennung und Transmutation von Actiniden: Partitioning. Reducing spent nuclear fuel radiotoxicity by actinide separation and transmutation: partitioning.
It is important to note that simply because a material is radioactive does not imply that it is not useful, perhaps even capable of accomplishing tasks that nothing else can do as well or as sustainably. Given the level of chemical pollution of the air, water and land, in fact, the use of radiation, in particular high energy radiation, gamma rays, x-rays, and ultra UV radiation may prove to be more important than ever, but that's a topic for another time...
The article notes that Marie Curie died from aplastic anemia and it is widely assumed, and most probably accurate, that her death was induced as a result of her work in the discovery and industrialization of a radium industry. What is not noted in these accounts is that she lived a relatively long life for her times, dying at the age of 66 in 1934. (She was born in 1867, in Poland.)
Her husband, Pierre Curie, was killed in a road accident. Had he not been killed, it is possible, as was the case of Madame Marie-Anne Paulze Lavoisier, the wife of the "discoverer" of oxygen, Antoine Lavoisier, one of the world's earliest scientific chemists, Madame Marie Skłodowska Curie's work would have been largely attributed to her husband, although they both received the Nobel Prize while he was still alive. (Marie Curie would later receive a second Nobel Prize, making her one of only three people, including Linus Pauling - chemistry and peace - and K. Barry Sharpless, the latter, winner of two prizes in Chemistry, asymmetric synthetic organic chemistry and click chemistry, who is still alive, and who I saw speak a few years back on the subject of how to do science.)
As an aside, the reason that we know as much as we do about the work of Antoine Lavoisier is the work of Marie-Anne Lavoisier, who was a great chemist in her own right. She kept records of her husband's (and her own) work, in which she was a professional as well as personal partner, after his execution by guillotine in the Great Terror of the French revolution.
I had the privilege of seeing Dr. Sharpless speak at the Museum of Science and Industry in Philadelphia, the web page of which includes a short biography of Marie Skłodowska Curie:
Marie Sklodowska Curie
If you find yourself in Philadelphia with some time on your hands, the small free museum is certainly worth a visit. It's near some of the Benjamin Franklin historical sites, which is appropriate, since Franklin was originally famous for his scientific work, before he went on to invent the United States, which lasted well over two centuries before falling because of an ignorant, uneducated, orange pedophile, something of an absurdity, but all the same true.
To return to Dr.Sklodowska Curie
One of the fun things to know about Marie Sklodowska Curie is that after her husband's death, she had an interesting, at the time controversial and scandalous, sex life, famously conducting a fairly well known affair with the married physicist Paul Langevin. Besides being a great scientist, one of the greatest of all time, she was rather attractive physically as a young woman, albeit being, especially in her time - although things are not all that much better in our times - a rare case where her public image generally disregarded her looks in favor of her mind.
Have a nice weekend.
If a plagiarized paper by an author who claims he didn't write it disappears from a journal's website with no notice...
...did it ever exist?
Journal silently removes paper for plagiarism, author claims identity theft
As a master’s student in 2011, researcher Silvia De Cesare published a paper in Implications Philosophiques analyzing a 20th century philosopher’s skepticism of the theory of evolution despite its compatibility with his philosophical views. Now with two doctorates — in ecology and in philosophy — De Cesare is a postdoctoral scholar at Utrecht University in the Netherlands and studies the relationships between evolutionary theory and the idea of progress.
In June last year, De Cesare learned that someone had published a version of her article in the International Journal of Applied Science and Research (IJASR) in 2020. The paper, a near-verbatim copy of De Cesare’s article apart from the omission of a few footnotes, listed Marcellin Lunanga Mukunda, of the University of Kinshasa in the Democratic Republic of the Congo, as its sole author. But Mukunda denies publishing the paper, telling us he had been hacked, or perhaps robbed, as an explanation for how his name appeared on the paper....
... IJASR has not posted a retraction notice on their website. The journal is not indexed in Scopus or Clarivate’s Web of Science. IJASR’s Publication Ethics page contains sections referring to itself as “Journal Sosioteknologi” and shares a significant portion of its text with the Journal Policies page of a journal by that name.
Mukunda, now an associate professor at the Higher Institute of Medical Techniques of Bukavu in the DRC, told us he did not submit the paper. “I have never published an article in the IJARS [sic] journal, which I consider to be predatory,” he said...
In Memoriam: The Scientific Legacy of Dr. Katherine T. Peter
The open source paper I'll reference in this post is this one: In Memoriam: The Scientific Legacy of Dr. Katherine T. Peter David M. Cwiertny, Edward P. Kolodziej, Gabrielle P. Black, John Kucklick, Ruth Marfil-Vega, Andrew D. McEachran, Benjamin J. Place, Jessica L. Reiner, and Alix E. Rodowa Environmental Science & Technology Article ASAP.
Dr. Peter was one of the discoverers of the fact that an antioxidant in tires, 6-PPD Quinone, when solubilized in rainwater or snow melt that flows into streams is responsible for the deaths of Coho Salmon and other fish species in streams and rivers near roadways.
She died recently as a young woman, from misdiagnosed cancer, the symptoms of which were attributed to her pregnancy with her only child.
Some excerpts:
...Kathy’s career started in the Department of Energy, Environmental and Chemical Engineering at Washington University in St. Louis. There, she conducted undergraduate research in the laboratory of Dr. John Fortner, broadly in the area of environmental nanotechnology. Her contributions led to her first peer-reviewed publication in ES&T Letters, exploring the photo-oxidation of hydrogenated fullerenes in water. (1) Kathy was an accomplished student, earning a perfect 4.0 GPA while attaining her Bachelor of Science in Chemical Engineering (valedictorian of the College of Engineering while double majoring in Spanish) and playing for the university softball team...
Kathy then pursued a Ph.D. in Civil and Environmental Engineering at the University of Iowa through support from the NSF Graduate Research Fellowship. At Iowa, Kathy continued her work on environmental nanotechnology, crafting her thesis around the application of electrospun nanofibers in point-of-use water treatment. Her work was motivated by the numerous challenges confronting Iowa’s private well owners, where over 300,000 vulnerable consumers fall outside of Safe Drinking Water Act protections and often need multifunctional, broad-spectrum purification technologies in a small device footprint...
...During Kathy’s first stint at CUW (2016–2019), almost the entire research group was focused on the compelling question of: what was killing Pacific Northwest coho salmon during fall rainstorms? To understand observations of recurrent acute mortality, the CUW lab was using environmental mass spectrometry tools to characterize and track the chemical composition of roadway runoff, urban stormwater, and associated receiving waters. This unifying theme and focus on discovery science initiated an integrated effort to apply comparative HRMS analysis to roadway runoff, small creek receiving waters, and associated treatment systems. It also motivated the development of source tracking and fingerprinting tools that could be used to compare and characterize water quality degradation and dynamics during the storm events where coho salmon would perish. This was all done in conjunction with parallel toxicology studies led by Dr. Jen McIntyre (Washington State University) and Dr. Nat Scholz (NOAA-NMFS).
Kathy quickly became an intellectual and organizational leader and driving force of many research and analytical studies to better understand urban water quality and associated chemodynamics. For example, CUW was working with citizen science and community groups to enable sampling of receiving waters where symptomatic coho salmon were observed during rain events. Kathy enhanced and initiated collaborations with several community groups, including Puget Soundkeeper, the Thornton Creek Alliance, and especially the Miller-Walker Community Salmon Investigation located on Miller Creek in Burien/Normandy Park WA, USA...
...Due to Kathy’s involvement, the water quality infrastructure and enthusiastic citizen science collaborations have led to multiple water quality and toxicology studies at Miller Creek which is representative of small, roadway runoff-impacted watersheds. After the initial 2018 Miller Creek publication, Kathy worked to define the chemodynamics of roadway runoff impacts on small riverine watersheds, demonstrating that tire chemicals can be abundant enough to be transport-limited environmental contaminants and defining exposure periods and sampling strategies needed to better understand transient water quality degradation during storm events. (13)...
Dr. Peters was an expert in "NTA" "nontargeted analysis" using high resolution mass spec.
All of humanity can be thankful that her too short life came to pass, and grieve that that life, well lived, ended too soon.
German energy policy moves from expensive stuff that doesn't work well to stuff that doesn't exist.
Um, OK...
Germany’s Merz: Nuclear fusion to make wind power obsolete
The participants in this week’s North Sea Summit in Hamburg committed to building 15 GW of offshore wind per year over 2031-2040. Country leaders including German Chancellor Friedrich Merz confirmed the goal of 300 GW on the so-called North Seas by 2050. At the same time, he apparently believes that wind turbines will begin to be dismantled much sooner!
Wind power is a “transitional technology” and it will be around for “ten, twenty, maybe thirty years,” Merz claimed, as quoted by Bild. He expressed confidence that Germany would put the world’s first fusion reactor online and estimated it would make electricity so cheap that no other generation methods would be needed.
Merz said repeatedly that the nuclear exit was a mistake
The country abandoned investments in nuclear power after the 2011 Fukushima disaster and prompted early closure of all reactors. Causing much controversy, especially during the recent energy crisis, the phaseout was completed in 2023.
In the campaign before last year’s elections, Merz called wind turbines ugly, adding that he’d like if they could eventually be taken down. He recently said the nuclear exit was a “huge strategic mistake” that caused “the most expensive energy transition in the entire world...”
Nuclear fission works, is cleaner and safer than coal, on which Germany now depends, and far more reliable and sustainable than so called "renewable energy." There is no evidence that fusion ever will drive a power plant.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 37,549