NNadir
NNadir's JournalA Brief Note on the Toxicology Associated With Hydrogen Fuel Cells.
Intellectually, and morally, in my view, the hydrogen fuel fantasy should have been, and should be now, a non-starter, simply because of the laws of thermodynamics, notably the 2nd law. Like other "bait and switch" fantasies connected with diversion from the facts that fossil fuels can be rendered sustainable (or eliminated by wishful thinking) - including but not limited to sequestration, wind, and solar - the use of hydrogen fuels will make things worse, not better.
I laid out, in some detail, the facts connected with the nature of hydrogen as a front for the fossil fuel industry in a rather long post here: A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
This is also laid out in the scientific literature in the case of China, to which fossil fuel salespeople and salesbots who write here and elsewhere often appeal here to rebrand fossil fuels as "hydrogen:"
Subsidizing Grid-Based Electrolytic Hydrogen Will Increase Greenhouse Gas Emissions in Coal Dominated Power Systems Liqun Peng, Yang Guo, Shangwei Liu, Gang He, and Denise L. Mauzerall Environmental Science & Technology 2024 58 (12), 5187-5195.
One of the "bait and switch" tactics used to greenwash fossil fuels by talking about hydrogen is to talk about hydrogen fuel cells for cars. Hydrogen fuel cells actually are commercial products occasionally used for back up power for things like cell phone towers, although they are hardly mainstream.
One of the major environmental risks garnering increasing attention, is the issue of PFAS - "per (and poly) fluorinated alkylate substances, so called "forever chemicals" - which are known to have profound toxicological profiles. I know, but I don't believe that most people know, that the issue of PFAS is very much connected with hydrogen fuel cells, and that the widespread use of these, for instance in cars, would make this already intractable problem far worse.
A paper on strategies to degrade these has appeared in the scientific literature in an open source paper that I came across in my general reading. It is here:
Oxidative Transformation of Nafion-Related Fluorinated Ether Sulfonates: Comparison with Legacy PFAS Structures and Opportunities of Acidic Persulfate Digestion for PFAS Precursor Analysis Zekun Liu, Bosen Jin, Dandan Rao, Michael J. Bentel, Tianchi Liu, Jinyu Gao, Yujie Men, and Jinyong Liu Environmental Science & Technology 2024 58 (14), 6415-6424
Again, the paper is open sourced, and will be generally arcane for people not familiar with scientific punctilios, but a relevant paragraph referring to fuel cells and the relationship to PFAS is this one:
Nafion, which is used in all commercial fuel cells, is the a fluoropolymer of PFAS compounds.
Two figures from the paper:
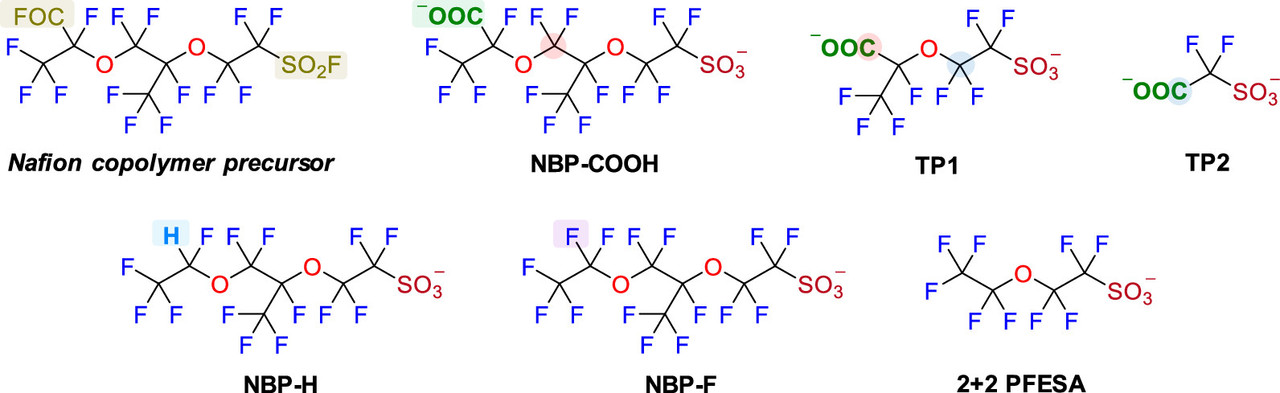
The caption:
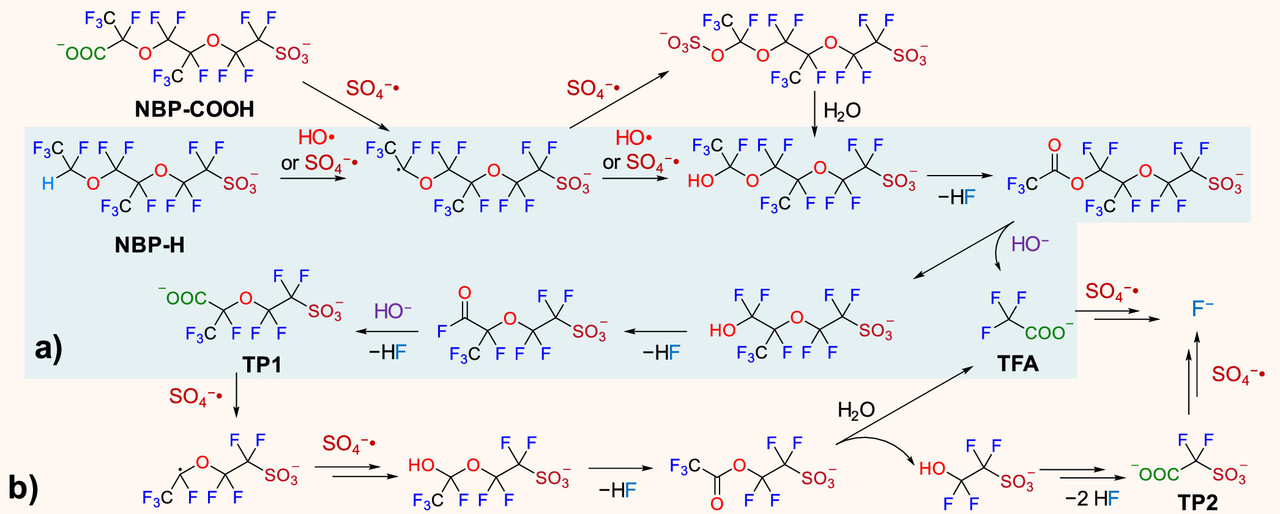
The sulfate and hydroxide radicals in this pathway require energy to create. I regard oxidative radicals of this type as the best pathway for mineralizing (destroying) PFAS. The type of energy ideal for this purpose is ionizing radiation, high energy radiation in the UV to gamma range. The energy for doing this is best obtained from radioactive materials, the easiest access to which can be obtained from used nuclear fuels.
Have a pleasant Sunday.
That scene in "My Dinner With Andre" About a Sexy Picture, and Looking at Pictures of My Wife When She Was Young.
Last week I wrote how my wife was going through a box of her late mother's pictures, and I found myself remarking on how beautiful she looked in a picture someone took of us at the North Rim of the Grand Canyon, just before we married, a picture that somehow ended up in my mother's-in-law collection and wasn't in ours.
My wife finally got around to going through her late Mom's photo albums.
A strange feature of the picture is that in it she actually looks younger than she was at the time; she was in her early twenties but looked like a teenager. By contrast, although I had hair and it was dark, and in the picture relatively long and wild in what may have been a wind, I looked older than I actually was; I was in my early thirties. (Because we looked as if the distance in our ages appeared to be larger than it was - my wife got "proofed" in restaurants and bars into her thirties - I used to get some dirty looks from time to time, as if I were an Epsteinish/Trumpish/Gaetzish sort of pervert.)
Well, in any case, I scanned the picture, and I've looked at it a number of times since doing so over the last week, fascinated by it. As I was doing so this morning, it suddenly struck me, looking at that picture of myself, that the man in the picture, me, didn't at that time know very much at all about the world; I was ignorant, even more arrogant than I am now, kind of smug, and although I was merely formally educated, but largely unacquainted with the deeper reality of the things to which my education merely exposed me.
Then I looked at my wife in the picture, and thought about who she was at the time, the Mona Lisa type smile she had on her face concealing the fact that she'd just emerged from a very unhappy upbringing and was possessed of a deep insecurity, and uncertainly - wisely I think - unsure whether it was actually a good idea to be with me, there and then.
In that beautiful movie My Dinner With Andre there's a scene where Andre Gregory discusses a picture of his wife, Mercedes "Chiquita" Gregory. From the script:
Script, My Dinner With Andre
The first time I went away with my (then future) wife, I took her to what was my favorite place in the universe, to Big Sur. We stayed in this beautiful small cabin in a grove of redwoods, a night I have never forgotten, and about which I often muse. I consider it the best night of my somewhat pathetic life up to that point - although in the years of marriage, many similarly beautiful moments would happily entail over and over again.
In the morning after that night in the cabin, we went on a hike up a small mountain in Pfeiffer Big Sur State Park, and we came upon a grove of pin oaks, and I took pictures of her up there in that grove.
There is one of those pictures in particular that has thrilled me ever since I took it and developed it almost 40 years ago, because in the picture, my wife has exactly the same expression she was wearing the very first time I saw her, the same pose, her legs stretched out, looking down almost shyly at her feet, detached from the outer world, but seemingly deep in an inner world, a kind of impenetrable look of something that could, should perhaps, be recognized as having an aura of buried pain, a resigned sadness strangely contrasting with a sublime physicality. In the Big Sur picture as opposed to my memory of first seeing her, she is sitting on a low branch of a massive oak the Big Sur grove, naked. I must have called that picture up a thousand times, but today, I find myself asking myself if I ever really saw it, I mean, saw it for real.
Of course, when I saw my wife for the first time, I was just another of the many puerile men possessed of less than noble erotic fantasies about her looks; I had no idea who she actually was. Of course, ultimately I did get to know her, and see her divorced from any kind of vanity, somewhat distressed about the somewhat dangerous situations into which her looks had gotten her although she managed to emerge largely unscathed. (Of course early on in our friendship, she could of suspected me of being another dangerous man of the type, but happily she didn't translate those things into suspicions of me or my intentions, although she might well have done so.)
Away from all that, the attentions of puerile men including me, she was always warm, kind, generous, funny and bright, surprisingly accessible and down to Earth, although the deeper things were kept to herself. I could not help falling in love.
But the point is, that in my admiration for these pictures of her youth, like Andre Gregory confessed in his own case, it's not act of seeing, but definitely an act of not seeing anything at all.
We were younger, better looking then, but frankly and honestly, as life winds down, I prefer who we are now. We hadn't lived at all then. We didn't know anything then. We've lived now. We know something, perhaps not a lot, but something now.
Most importantly, something we know now is what it is to have lived at all. That matters.
BWXT announces nuclear manufacturing plant expansion
BWXT announces nuclear manufacturing plant expansionAn excerpt:
BWXT is headquartered in Virginia and has 14 operating sites in the United States, Canada, and the United Kingdom. Its joint ventures provide management and operations at a dozen U.S. Department of Energy and NASA facilities.
What they are saying: “Our expansion comes at a time when we’re supporting our customers in the successful execution of some of the largest clean nuclear energy projects in the world,” said John MacQuarrie, president of commercial operations for BWXT. “The global nuclear industry is increasingly being called upon to mitigate the impacts of climate change and increase energy security and independence.
Premier of Ontario Doug Ford said, “We’re thrilled to see BWXT expand its footprint and create hundreds of new jobs in Cambridge. As our province continues to lead the future of nuclear energy, the company’s investment will help provide Ontario families and businesses with access to clean, reliable, and affordable electricity for generations to come...”
I added the bold.
This won't go over well in the cults that drive the so called "renewable energy"/fossil fuel nexus, but for humanity as a whole this is a good thing, building back better the nuclear manufacturing infrastructure destroyed by fear and ignorance, thus leaving the planet in flames.
Nuclear systems can do more than provide families "with access to clean, reliable, and affordable electricity for generations to come," although there's certainly nothing wrong with that. It's nice to see people thinking about future generations, something certainly ignored by advocates of the so called "renewable energy"/fossil fuel nexus, where future generations are treated with contempt.
Personally, though, I'm opposed on thermodynamic grounds, to the "electrify everything" bad idea that's gaining unwarranted popularity, but we have to restart somewhere. (Electricity is by its nature, thermodynamically degraded.) With the planet already in flames, notably with Canada for one subset of the climate burned regions, this comes under the rubric of "too little, too late" and is hardly enough, but the world seems to be overcoming the triumph of antiscience antinukism to try to save what is left to be saved and perhaps, even restore some of what can be restored.
Ontario is rising as a center of going nuclear against climate change, something of which citizens of the Province should be proud.
Non Nuclear Portions of Wyoming's Kemmerer Nuclear Power Plant to Start Construction This Summer.
TerraPower submits Natrium construction application to the NRCExcerpts:
TerraPower purchased land in Wyoming near one of the state’s retiring coal plants to deploy its Natrium sodium fast reactor technology. Kemmerer Power Station Unit 1 would operate a 345-MW sodium-cooled reactor in conjunction with molten salt–based energy storage...
...A closer look: Natrium will use liquid sodium as a coolant instead of water. According to TerraPower, the reactor features improved fuel utilization, enhanced safety features, and a streamlined plant layout that will require fewer overall materials to construct. Because of the reactor’s storage technology, it can boost the system’s output to 500 MWe for more than five and a half hours when needed to meet additional grid demand...
... Support for the project: Natrium is one of two advanced reactor demonstration projects selected for competitive funding through the Department of Energy’s Advanced Reactor Demonstration Program. (X-energy’s Xe-100 is the other.) TerraPower received $1.6 billion in funding from the Bipartisan Infrastructure Law signed by President Biden in November 2022, which is to be used to ensure the completion of the plant. The company has also raised more than $1 billion in private funding.
Last month, TerraPower announced the second round of contracts for long-lead suppliers supporting the development of the Natrium reactor.
I'm not a sodium coolant kind of guy myself, but this reactor has features of the "breed and burn" concept which will allow it ultimately to run on depleted uranium, if I understand the design well.
Light a Candle, an Innovative Burn Up Strategy for Nuclear Reactors. (Hiroshi Sekimoto)
This will help to generate plutonium necessary if we are to have any hope of addressing climate change.
Dr. Kathryn Huff Is Leaving DOE, Praising the Administration as She Goes.
After serving her country for three years, Dr. Kathryn Huff is leaving DOE:
Kathryn Huff stepping down from DOE Nuclear Energy post
Excerpt:
“Serving in this capacity has been an unparalleled privilege, and I’m immensely grateful for the opportunity to have worked alongside you--the dedicated and talented public servants in Nuclear Energy, in DOE, and across the Biden-Harris Administration,” Huff wrote in an email announcement to colleagues last week. “I chose this timing to enable the smoothest transition back to my professorship at the University of Illinois at Urbana-Champaign where my beloved research, students, husband, and dog await...”
... “Reflecting on the past three years, I’m astonished by the tangible progress the U.S. has made in nuclear energy,” Huff said in her note to colleagues. “Reactors once destined to shut down now have a role in our 2035 and 2050 goals, new reactors are coming online, and the commercialization of advanced reactors has begun. We’re also securing the front end of the nuclear fuel cycle, restarting a consent-based approach to spent nuclear fuel management, and expanding international cooperation on peaceful nuclear technology. Perhaps most notably, the U.S. joined a groundswell of other nations in committing to triple nuclear energy capacity by 2050 (to) address the climate crisis and improve energy security.”
She added, “I will, of course, continue to contribute to the advancement of nuclear energy however I can, for as long as I can, from wherever I am.”
I understand her reasons for leaving, but I'm a little sad to see her go. She was, to my mind, a star in the Biden-Harris administration, a high powered academic scientist bringing high level expertise to government. It does seem her accomplishments in the move building back better our nuclear power infrastructure have been significant, the most significant since Steven Chu, in the Biden administration started us on the now nearly complete path to the Vogel reactors.
A Recent Thread Not Removed or Deleted Is Not Appearing In the Science Forum Titles.
It refers to an article in a scientific journal.
The post is this one: Nature Energy: An Estimate of the Death Toll Associated with a US Nuclear Power Phase Out.
Oddly it seemed to have received one of 7 recs after disappearing. (I can access it through "My Posts." )
???
Nature Energy: An Estimate of the Death Toll Associated with a US Nuclear Power Phase Out.
The paper to which I'll refer in this post is this one: Freese, L.M., Chossière, G.P., Eastham, S.D. et al. Nuclear power generation phase-outs redistribute US air quality and climate-related mortality risk. Nat Energy 8, 492–503 (2023).
I'm logged into my Nature account; apparently the paper is not open sourced.
Some excerpts:
Of course, there is in this paper lots of reference to so called "renewable energy" and the usual soothsaying that goes with it, although the reality, after the expenditure of a little over 4 trillion dollars in this scam in the period between 2015 and 2023, it has done essentially nothing more other than to accelerate the rate of climate change. After nearly half a century of such soothsaying there is little reason to expect the result will be any different.
The article discusses this thing called "reality:"
These recent shut-downs include the Indian Point Energy Center second reactor, which was shut down in April 2021 because of environmental and safety concerns due to its proximity to New York City11. Browns Ferry and Sequoyah nuclear power plant shut-downs in 1985 led to increased coal use12, as determined by regressions comparing power plant level production in the Tennessee Valley Area before and after the nuclear plant closures. Using similar regressions to assess generation by plants before and after the San Onofre Nuclear Plant (California) shut-down in 2012, ref. 13 found nuclear power plant closure led to increased gas use, as well as increased costs of electricity generation. Recent work has shown that phase-out of nuclear power from 2011 to 2017 in Germany led to replacement by fossil fuels14.
Many antinukes whine when I point out that they just don't give a rat's ass about climate change. The data referenced in the two paragraphs just posted makes this very clear. Even though they can't actually produce numbers that suggest that there is any form of energy with a risk as low as that of nuclear energy, they swear up and down they give a shit about climate change before launching into the usual selective attention balderdash about how "dangerous" nuclear power is. (Compared to what? Climate change?) They're lying.
The article continues:
Previous work has only addressed subnational-level response to nuclear power shut-downs or has quantified regional and globally averaged avoided mortalities from nuclear power use. Using the InMAP reduced form model, ref. 19 found that the shut-down of three nuclear power plants in the Pennsylvania–New Jersey–Maryland region led to increases in PM2.5 resulting in 126 additional mortalities. Another study5 quantified the global historical prevented mortalities and CO2 emissions due to historical and potential future nuclear power generation, using average mortality rates and CO2 emissions rates by electricity type. They project mortalities and CO2 emissions based on energy projections by the UN International Atomic Energy Agency out to 2050, finding that 4.39–7.04?million deaths would be prevented by using nuclear power, rather than fossil fuels, due to lower emissions of air pollutants. Previous work also has not consistently accounted for the potential growth of renewable energy, which has been shown to replace the use of fossil fuels20.
The study breaks down the ethnic distribution of people likely to be killed by nuclear shutdowns.
A figure from the paper:
Fig. 3: Distribution of exposure and mortalities by race and ethnicity for each county in no nuclear.
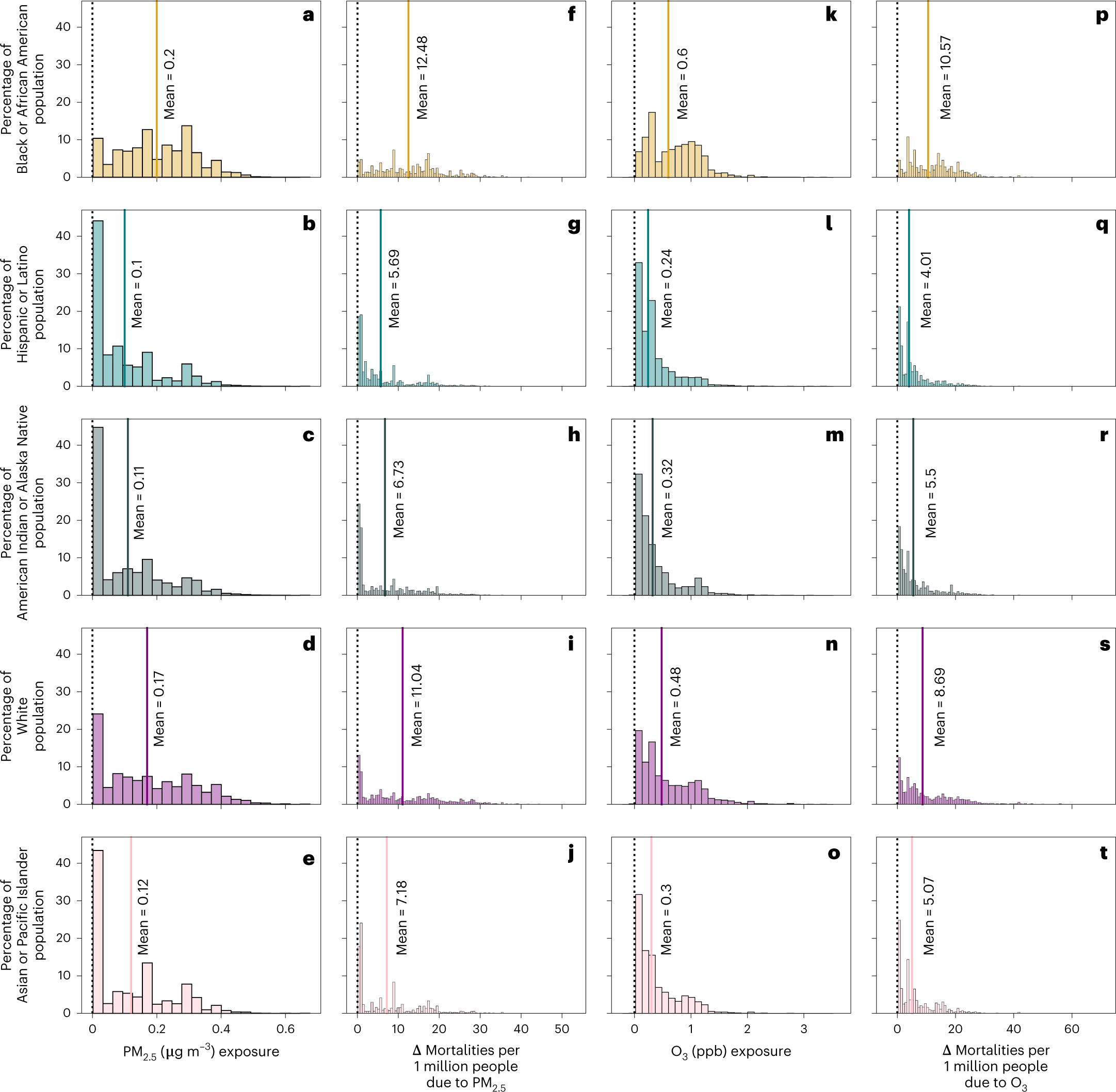 ?as=webp
?as=webp
The caption:
More text:
I was banned some years back at another website, one nowhere near as good as DU, for making the true statement that opposing nuclear energy kills people, referring to James Hansen's seminal 2013 paper stating as much:
Prevented Mortality and Greenhouse Gas Emissions from Historical and Projected Nuclear Power (Pushker A. Kharecha* and James E. Hansen Environ. Sci. Technol., 2013, 47 (9), pp 4889–4895)
Truth has a way of producing scorn. So be it.
The full paper can be accessed at the link in a good University library or by a Nature+ subscription. The authors are from MIT.
Have a nice evening.
My wife finally got around to going through her late Mom's photo albums.
The photos are circulating between the sisters in the order of birth, my wife being second. We're supposed to select the ones we want and pass them on to the next sister. My wife, considerately, isn't taking many she believes her younger sisters would want.
The photos go back to the very early 20th century and even a few from the 19th century. We're not entirely sure who all these people were, but its fun to speculate from the family stories we know. They say you die twice, once physically, and then when no one remembers who you were.
What is striking to me is that my mother-in-law had quite a few pictures of my wife and I from the period before we married and some from the early years of our marriage. This is somewhat surprising since I don't think my mother-in-law particularly liked me at the time, although we worked out our problems eventually.
I guess I looked slightly less dumpy back then, but damn! I'd forgotten how drop dead gorgeous my wife was physically. (She's still drop dead gorgeous, but in a different way.) I do remember all the men hitting on her, but she stuck with me somehow. I was very lucky.
There's one of us standing on the North Rim of the Grand Canyon - a stranger must have taken it for us - during our trip moving out to California that just blows my mind. I sure looked happy, and I sure know why.
I recall hearing from a colleague that the guys who worked for me at one of the jobs out in California that I held early in my marriage asked him what the hell a woman that beautiful was doing with the likes of me. I kind of laughed it off back then, but I see what they were wondering about.
I don't think I have an answer.
We've been together 40 years, and I still don't have an answer.
Of course, her looks have very little to do with who she is. It is the latter, who she is, that matters in the end.
I just saw a live performance by the greatest drummer I have ever seen.
Dafnis Prieto
Various awards include a 2011 MacArthur Fellowship Award; a GRAMMY Award and a Latin GRAMMY Award nomination for Best Latin Jazz Album for Dafnis Prieto Big Band Back to the Sunset in 2018; a GRAMMY Award nomination for Best Latin Jazz Album for Dafnis Prieto Sextet Transparency in 2021; a GRAMMY Award nomination for Best Latin Jazz Album for Absolute Quintet, and a Latin GRAMMY nomination for “Best New Artist,” in 2007; and “Up & Coming Musician of the Year” by the Jazz Journalists Association in 2006. Also a gifted educator, Prieto has conducted numerous master classes, clinics, and workshops throughout the world. He was a faculty member of Jazz Studies at NYU from 2005 to 2014, and in 2015 became a faculty member of Frost School of Music at UM (University of Miami), where he directs the esteemed Frost Latin Jazz Orchestra.
As a composer, Prieto has created music for dance, film, chamber ensembles, and most notably for his own bands ranging from duets to big bands, including the distinctively different groups featured by nine acclaimed recordings as a leader: About The Monks, Absolute Quintet, Taking The Soul For a Walk, Si o Si Quartet-Live at Jazz Standard, Dafnis Prieto Proverb Trio, Triangles and Circles, Back to the Sunset, Transparency, and Cantar. In 2022 Prieto premiered a new work for Latin band and string orchestra — Tentación — performed by People of Earth with the Louisville Orchestra, the Los Angeles Philharmonic, the New World Symphony, and the Britt Festival Orchestra. He has received new works commissions, grants, and fellowships from Chamber Music America; Princeton University; Jazz at Lincoln Center; Museum of Modern Art; Whitney Museum; National Association of Latino Arts & Cultures; Jerome Foundation; East Carolina University; Painted Bride Art Center; Meet the Composer; WNYC; the Louisville Orchestra, the Britt Festival Orchestra, New Music USA, Hazard Productions, and People of Earth; and the Metropole Orkest...
Liner notes from the Princeton Jazz Festival.
I have been waiting for about 20 years for this paper to appear.
Listen folks, most of what one hears here and elsewhere about addressing what is possibly the most serious issue before humanity since humanity passed out of Africa to the larger world, climate change, is pure bullshit.
I cannot be dissuaded from taking this position, not by people who insipidly mutter "Fukushima" or "Chernobyl" of (even more stupidly) "Three Mile Island," not by people who think that the existence of plutonium inevitably will lead to nuclear war. The entire history of nuclear war, has not killed a tiny fraction of the people killed by fossil fuel wars; the entire history of the accumulation of so called "nuclear waste" has not killed as many people as will die this afternoon from fossil fuel waste, and has killed each and every afternoon of this century.
The last hope of humanity is to convince ourselves to consider things as the are and to prioritize them over the things we imagine through a prism of fear and ignorance.
Because so many of us are in our own ways as ignorant as right wing anti-nuke and antivaxxer Bob Kennedy the 2nd, here's where we are as of this morning with respect to the concentrations of the dangerous fossil fuel waste CO2:
Week beginning on April 07, 2024: 425.90 ppm
Weekly value from 1 year ago: 422.68 ppm
Weekly value from 10 years ago: 401.36 ppm
Last updated: April 13, 2024
Weekly average CO2 at Mauna Loa (Accessed 4/13/24)
Back in the late 1950's and early 1960s, the American scientists ran an nuclear reactor known as the LAMPRE, (Los Alamos Molten Plutonium Reactor Experiment).
It was in the era of dynamic creativity in the development of nuclear energy which has been described beautifully in a monograph by the former director of ORNL, Alvin Weinberg, who died at the age of 91 in 2006: The First Nuclear Era.
That dynamism was quashed by appeals to fear and ignorance; it was an intellectual infrastructure that was destroyed along with the manufacturing and operational infrastructure in nuclear energy with the result that the planet is in flames.
Now we are in the "build back better" phase of the nuclear intellectual infrastructure; at least I hope we are. To build back, we must reach back to what was lost.
For many years I have in my Google Scholar alerts, a "liquid plutonium" search term; because I despaired of anyone anywhere ever looking as deeply into molten metal fuels. I badger my son, a nuclear engineering Ph.D student, about it from time to time, although his research interests are in nuclear materials as opposed to fuels. (In molten fuels, materials science is indeed critical, liquid plutonium is an excellent solvent. It dissolves steel and many other metals.)
Anyway, this morning the paper I was hoping would show up did.
Here's the link: Molten Fuel Fast Reactor: Concept of Core, Fuel Efficiency, and Safety (V.S. Okunev, 2024 6th International Youth Conference on Radio Electronics, Electrical and Power Engineering (REEPE))
The abstract:
I have downloaded the full paper and will badger my son by sending it to him so it may remain in his mind through his career, a career I hope will be dedicated to saving what is left to save and restoring what can be restored.
As my life winds down in the awful times through which I have lived, observing in a peculiar way the realities, my hope for the future has been challenged. I often think that anything we do will be too little, too late. I have enough hope left to hope I'm wrong.
Dr. Okunev is a Russian scientist, and thus lives in what is now a pariah state. Even in a dark world, there can be places in which light can emerge, and to my mind Dr. Okunev is just that, a light in darkness. I hope this paper gets some attention.
Have a pleasant weekend.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,580