NNadir
NNadir's JournalLet's Not Overthink This.
The Editorial in the Current Issue of Science.
Let's Not Overthink This. H. Holden Thorpe.
Science and political communication scholar Kathleen Hall Jamieson of the University of Pennsylvania believes that it is wrong to construe the election in such simple terms. “Science was not on the ballot,” she said in a recent conversation. When viewed in the heat of the battle, she says, the 73 million people who voted for Trump may seem to have been rejecting science, but many of them live in areas of the country that had, until recently, barely experienced the COVID-19 outbreak. Now they are getting it full blast. Others simply believed that the health of the economy should not be jeopardized by what they saw as a draconian pandemic response. As for the fight against climate change, many people feared that their livelihoods would be threatened by calls for a major move away from fossil fuels. Add to that the millions of people whose religious beliefs enjoin them from appreciating the beauty and power of the theory of evolution. There is not one great horde of Americans (many of whom happen to be Trump supporters) who are anti-science. It is a mixture of people who, for personal reasons, resist facts that challenge their thinking...
...Most people don't think about the biology of the promised coronavirus vaccine any more than they marvel at how the theory of general relativity is used by satellites to guide them as they navigate with Google Maps.
The periods of high American enthusiasm for science have all coincided with great triumphs for science such as the Moon landing or the polio vaccine. But after all this excitement, science moved off center stage, and the scientists could quietly go back to work. We're on the cusp of a similar cycle...
No comment from me other than to say that it's not just Trump-cultists who are unwilling to challenge their "thinking..." such as it is.
Metal Oxide Sorbents for the Separation of Radium and Actinium
(Note: This post, and many of my earlier posts in this space, contains some graphics which may not be accessible to Chrome users because of a recent upgrade to that browser, but should work in Firefox and Microsoft Edge. When my son has time, he will adjust the file system for a website he's building for me to make these graphics usable in Chrome, but he seldom has that much time on his hands. Interested parties, should they exist, can still read my posts including the graphics, but regrettably must use a browser other than Chrome. Apologies - NNadir)
The paper I'll discuss in this post is this one: Metal Oxide Sorbents for the Separation of Radium and Actinium (M. Alex Brown Ind. Eng. Chem. Res. 2020, 59, 46, 20472–20477)
One of the more interesting things about anti-nukes is the deep abiding concern they show for uranium ore tailings - which contain radium, most of which is Ra-226, the natural decay product of uranium-238, with a nearly vanishing fraction consisting of radium-223, from the uranium-235 decay chain.
On the other hand these same anti-nukes are spectacularly disinterested in a much larger source of radium that is associated with flowback water from natural gas fracking operations, particularly in the Marcellus Shale, which is a uranium formation. The same process that extracts natural gas, also extracts radium, and the flowback water is dumped right on the surface, with very little effort to control it, any more than there is any effort to contain the dangerous combustion combustion waste of dangerous natural natural gas, dangerous carbon dioxide.
Anti-nukes couldn't care less about dangerous natural gas, of course, since without access to it, the so called "renewable energy" industry would be exposed for the scam it has always been when the lights, refrigerators, theaters, home, public and surgical all go dark because the wind isn't blowing and the sun isn't shining. The Trump scale lie that's told about this crime against the future, burning natural gas, is that it's "transitional."
This is absurd, since the use of dangerous natural gas has continuously been growing at a vastly faster rate all through this century. (This is in terms of energy and not the often disingenuous and fraudulent appeal to peak capacity without reference to total capacity utilization, which for wind and solar is absurdly low, and thus materially wasteful, this while having no relation to demand.)
I have argued that the uranium (and thorium) already mined is sufficient to provide all of humanity's energy needs for centuries, at least in "breed and burn" scenarios, many of which are ready for commercial application, and thus uranium mining is not really necessary, and arguably will never be necessary again, at least in a world where attention to risk and the environment, and the facts of engineering are not subjects of derision and contempt, both on the political right and quite frankly, on the political left as well, albeit with different foci.
Because air pollution kills millions of people every year, and because the death toll from extreme heat is rising dramatically, the death toll associated with nuclear operations - which is clearly non-zero - is trivial compared with all other forms of energy. Nuclear energy, where it operates, saves lives. The argument that any or every nuclear death to which excessive attention is paid excuses millions of other deaths not associated with radioactivity which are ignored is not merely stupid; it is unethical in the extreme. It is in fact, equivalent that the two deaths at Bengazi outweigh the hundreds of thousands of Covid deaths, an argument the Republican party unashamedly makes much to the disgust of the entire civilized world.
Nuclear technology saves lives in other ways, most famously associated with medicinal use, both in imaging and in therapy and sometimes in the combined practice known as theranostics.
Radium-226, which has a half-life of about 1600 years, can be transmuted, in a neutron flux into the rare element actinium-227, which has a half-life of 21.77 years. This is the only naturally occurring actinium isotope that is present in large enough quantities in natural sources (uranium ores) to be isolated, but doing so is extremely expensive. Actinium-227 is too long lived to be of much use in medicine, although it would be an excellent fuel for a thermoelectric generator in bulk quantities, but the isolation of radium from either uranium mine tailings or flow back water itself is fairly expensive.
There has been a lot of interest however in another isotope of actinium, Ac-225, which has a half life of ten days and which is a powerful alpha particle emitter. Since alpha particles do not travel very far in matter, this means that any destruction associated with interaction with matter is highly localized, something that is clearly desirable in for instance, cancer treatment using an expedient like an ADC, an antibody drug conjugate, where the antibody has a CDR (complementarity determining region) associated with cell surface displays unique to cancer cells.
This paper is about Ac-225.
From the introduction:
Regardless of the production channel and as 225Ac is scaled toward curie quantities, it must be considered that increased alpha dose rates could detrimentally affect the chemical processing of the targets and products. The separation of Th targets, Ra, Ac, and fission fragments have been executed using commercial ion-exchange and extraction chromatography resins.(11?14) However, the alpha-destructive nature of irradiated Ra and Ac can incur radiological damage on conventional organic/silica-supported resins.(15) It is well documented that mCi quantities of these isotopes (particularly Ra and subsequent daughter products) can effectively destroy such resins to the point that in some cases the material can no longer be recovered from a column.(16?20) The estimated daily dose of 20 mCi 225Ac (including daughter products) to 100 mg of cation-exchange resin is 2 × 108 cGy.(21) The reported absorbed dose threshold of cation-exchange resins is 108 cGy, after which diminished performances have been observed.(16) Further, curie quantities of 225Ac will certainly degrade polyvinyl supports and introduce organic impurities that could contaminate the product and ultimately affect chelation chemistry.
There may be advantages in using metal oxide sorbents (frequently used in high-performance liquid chromatography)(22) considering their high radiation stability and widespread application in the recovery and purification of fission 99Mo...(23)
...The focus of this work was to investigate if inorganic-based normal-phase sorbents can be used as a platform to retain selected +2 and +3 cations—starting with Ba and La nitrate surrogates then onto to 228Ra and 228Ac tracers.(30)
In this case, the authors isolated Ra-228 from aged isolated thorium using solvent extraction procedures with a TOGDA complexing agent. Radium is a decay product of the thorium decay chain as it is in uranium-235 decay chain. Radium-228, which has a half-life of 5.75 years, decays by beta emission to Ac-228, which has a half-life of about 6 hours. Thus this system is a surrogate for the production of Ac-225 by either Ra-226(n,2n)Ra225 -> Ac-225 reactions or Ra-226(p,n)Ac-225 reactions.
To model this system, given the difficulty of isolating Ac-228 and Ra-228, Barium/Lanthanum was used to model these reactions. The authors then attempted separation using metal oxides.
Some pictures from the text showing some results:
Separations on titania and alumina, the oxides respectively of titanium (as marvelous TiO2) and aluminum:

The caption:

The caption:
The surrogate barium/lanthanum system:

The caption:
The real thing:

The caption:

The caption:
It is important to note that all of the radioisotopes mentioned in this paper occur naturally. When one fissions a naturally occurring nuclide, either as a transmutation product (Th-232 -> U-233 or U-238 -> Pu-239) or U-235 which occurs naturally, one eliminates all future members of the decay series. Although used nuclear fuel is highly radioactive owing to the short half-lives of most fission products, it can be shown, that in roughly 1000 years of continuously recycled nuclear fuel, the total radioactivity associated with nuclear fission energy will actually be lower than the natural radioactivity contained in radioactive ores of uranium and thorium. (This may or may not be a good thing.)
The conclusion of the paper:
Despite the rising Covid-19 numbers, I trust you will be safe and well and able to enjoy the many wonderful privileges of being alive. I wish you the happiest of Thanksgivings!
Omen Ranger.
Ice, Climate, Clathrate Physics and Greenhouse Gases.
(Note: This post, and many of my earlier posts in this space, contains some graphics which may not be accessible to Chrome users because of a recent upgrade to that browser, but should work in Firefox and Microsoft Edge. When my son has time, he will adjust the file system for a website he's building for me to make these graphics usable in Chrome, but he seldom has that much time on his hands. Interested parties, should they exist, can still read my posts including the graphics, but regrettably must use a browser other than Chrome. Apologies - NNadir)
The paper I'll discuss in this post is this one: Lattice Dynamics Study of the Thermal Expansion of C3H8-, CH4-, CF4-, CO2-, Xe-, and N2-Hydrates (Rodion Belosloudov et al., Energy Fuels 2020, 34, 10, 12771–12778).
The Montreal Protocol demonstrated that in the case where there are governments of good will, it is possible for humanity as a whole to address serious environmental issues. The ban on the production and use of chlorofluorocarbons has slowed the destruction of the ozone layer, although threats still exist to it, notably nitrous oxide, concentrations of which are rising. The Montreal Protocol required the replacement of the CFC's - chlorofluorocarbons - with HFC's hydrogen fluorocarbons. Like the CFC's, the HFC's are all powerful greenhouse gases, but they do not participate in the destruction of ozone appreciably. Because the carbon fluorine is very strong, however, these compounds are very persistent, and degrade very slowly. A related problem, less involved with the atmosphere, but very much a concern in water solutions, including the water solutions making up living things, is the problem of perfluorinated alkyl acids and perfluorinated polymers. The most familiar example of a perfluorinated polymer is Teflon; but other examples, notably Nafion, which is used in hydrogen fuel cells, exist.
The strength of bonds determines the energy required to break them, and their breakage is necessary for them to degrade. The Planck relation defines the energy of electromagnetic waves.
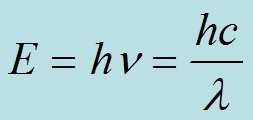
From this relationship one can readily determine that the required energy to break carbon fluorine bonds is found only in UV and shorter wavelength electromagnetic waves: Those in the x-ray and gamma region. Radiation with this energy is only found in the upper atmosphere, at least in the case where there is a functional ozone layer. On the earth's surface, radiation at this energy level must be obtained either using energy intensive accelerators - in the case of organofluoride destruction, electron beams - high intensity UV lamps, or readily available radioactive materials.
One of the most potent atmospheric greenhouse gases is tetrafluoromethane, CF4, which is discussed in the paper referenced at the outset. The chief industrial sources of this gas, which has a global warming potential, mole for mole, that is well over 6,000 times greater than carbon dioxide and an atmospheric lifetime of more than 10,000 years, is the production of aluminum, followed by silicon refining. The Hall-Heroult process by which aluminum is made is an electrochemical process in a molten fluoride based salt, cryolite, and the anodes in the electrochemical cell - ironically called a "green electrode" - is made of petroleum coke compressed into a composite solid using petroleum or coal tars (asphaltenes). During the electrochemical reduction of aluminum, these electrodes are sacrificial, yielding carbon dioxide and, from small amounts of fluorine produced, carbon tetrafluoride, both of which are vented to the atmosphere.
Anyone familiar with my writings will be aware that I strongly favor the reprocessing of used nuclear fuels, for which many processes are known; some of which I have discussed in this space. It's clear to me that even better processes than those currently utilized industrially, as well as many that have been explored at various depths, are quite possible. If someone were to ask me - no one will - what I thought would represent the most sustainable approach to recovering valuable materials used nuclear fuels, nuclear reprocessing, for those fuels historically accumulated for more than half a century, solid phase fuels, I would suggest electrochemical approaches, in some ways similar to the Hall-Heroult process, and the marvelous recently discovered FFC Cambridge process (which is chloride, and not fluoride based.) In at least one process I've dreamt up, the removal of dilute water from the reaction matrix might end up generating small amounts of CF4 in the case where carbon based electrodes were utilized. (This may not be necessary however.)
I favor closed cycles for all materials, not just nuclear materials, and I believe that human technologies should be required to provide for a safe and sustainable world for future generations, that is that each generation should leave the planet in better shape or at least the same shape in which they found it. This is obviously an approach that my detestable generation has failed to embrace. When I consider a process therefore, it is ethically incumbent upon me to consider the environmental fate of side products such as CF4. As stated above, if the task is to clean up destroyed or damaged matrices, one should also understand how to permanently destroy toxic materials, rather than playing a shell game involving various kinds of dumps, all of which will eventually leak, with varying impact on the health of humans and the planet. If the atmosphere and/or waterways are the effective dumps - and this is the general current practice for vast amounts egregious byproducts of human industry the problem of isolation and/or destruction is an enormous engineering problem, technically feasible perhaps, but certainly not cheap or easy. Right now this disposal CF4 and the HFC’s involves using the atmosphere as a dump, a practice that will greatly impact all future generations, and indeed, all life forms on this planet.
The destruction of atmospheric HFC's and residual CFC's is chemically similar to the problem of fluorocarbon derivatives in water, notably PFOS, PFOA, perfluorooctanoyl sulfonate and perfluorooctanoic acid respectively, as well as other fluorinated carbon compounds in as much as they both involve reactions with water as a reactant, water in the vapor phase in air. However because they are in very different matrices, water and air, the destruction pathways differ in their mechanisms in important ways. I have spent a long stretch of my adult life thinking about his problem, especially because I have considered the generation of CF4 in the reprocessing of nuclear fuels. This is true even though the high energy to mass ratio of nuclear fuels, the precise reason that they are environmentally superior to all other forms of energy generation, means that, even in an ideal totally nuclear powered world, the generation of CF4 would be trivial when compared to its generation in the aluminum and silicon industries. The question of destroying aqueous fluorocarbons is also related to my interest in nuclear fuels, not so much because reprocessing nuclear fuel would or could generate such compounds in water, but because the radioactive materials within them represent excellent tools for solving the otherwise intractable and growing problem they represent without expending vast amounts of climate destroying energy.
The radiation induced cleavage of a chemical bond can occur in two ways, heterolytically and homolytically. The heterolytic cleavage of a bond results in two ions, one positively charged and one negatively charged. Homolytic cleavage results in the production of two "free" radicals, a radical being a species with an unpaired, and thus highly reactive, electron. Although radiation induced heterolytic cleavage was historically important in techniques like mass spectrometry where Cf-252 sources were involved, modern techniques like electrospray ionization have supplanted them except in rare cases such as mass spectrometers on space probes. As far as the destruction of fluorinated hydrocarbons in water are concerned, the heterolytic cleavage of water is a non-event: Water self-ionizes spontaneously, accounting for the pH of pure water, which is 7. This self-ionization does not result in the appreciable destruction of fluorinated compounds. In both the gas phase, air, and the water phase, liquid water, radicals are responsible for the degradation of fluorinated carbons.
I discussed, a some length in the desultory way I am wont to discuss things, the decomposition of fluorinated carbon compounds here:
A Nice Scientific Review Article on the Destruction of Persistent Perfluoroorganic Pollutants.
(I remarked back then that I believed that trifluoromethanol could be synthesized without providing a reference. It turns out that it has been synthesized, albeit in a solution of HF and fluorophosgene:
Convenient Access to Trifluoromethanol (Christe et al., Angew. Chem. Int Edition, Volume 46, Issue 32 August 13, 2007 Pages 6155-6158). I'm not sure that "convenient" is exactly the word I would use for this chemistry. Fluorophosgene and pure condensed HF are very dangerous reagents, although this work was performed at the Loker Lab at USC where they worked with Nobelist George Olah of "super acid" fame. One isolation of trifluromethanol relied, in fact, on a super acid salt generated by the reaction of SbF5 with fluorophosgene. Interestingly it can also be obtained in the fascinating eutectic HF/CsF system with which I've been fascinated for many years.)
Anyway.
In my thinking as I wrote that post, I assumed that the pathway for the radiolytic destruction of CF4 would take place in the upper atmosphere where UV radiation would break a carbon fluorine bond, yielding a CF3٠ radical, which would then decompose following the mechanism shown in this paper, Fluorocarbons in the global environment: a review of the important interactions with atmospheric chemistry and physics (McCulloch, Journal of Fluorine Chemistry, Volume 123, Issue 1, 1 September 2003, Pages 21-29):

This pathway I find satisfying, in the sense that in a radiation field the greenhouse gas is irreversibly destroyed upon neutralization of the HF with a group II element oxide, generally calcium.
However, this pathway would not be found in water, where the reduction of CF4 would take place via "free" (solvated) electrons. It never occurred to me that CF4 might be soluble in water. In water, ? radiation generates ٠OH and ٠H radicals but the ٠OH radical in the presence of "free" electrons is rapidly converted to the OH- ion, and thus the CF3٠ radical can only react with ٠H radical to generate HCF3, fluoroform, the fluorine analogue of chloroform.
Unlike chloroform which is a liquid, albeit a volatile liquid, fluoroform is a gas, and in fact, a gas that is a more potent greenhouse gas than CF4 itself, with a 100 year global warming potential almost 15,000 times that of carbon dioxide, more than double that of CF4. Fluoroform has been released to the atmosphere as a side product in the synthesis of Teflon. It is also a refrigerant, where it is known as HFC-23, used as a replacement for the CFC’s, most commonly in automotive air conditioners. It is used also to etch silicon wafers in the semiconductor industry, albeit in a semi-destructive fashion.
It is well known that under pressure at low temperatures, methane forms "clathrates" in water, also known as hydrates. A clathrate, is an array of molecules forming a cage around a different molecule. A water cage if formed when water molecules assemble into the fused tetrahedral array that is characteristic of ice. Methane hydrates are heat sensitive, and whether they are deliberately mined for provide dangerous natural gas, our outgas because of rising temperatures. As such, whether mined our outgassed as a function of the rising temperatures of the planet, these hydrates represent a very real climate threat.
It appears that tetrafluoromethane forms water clathrates as well. The paper cited at the outset of this discusses clathrates of two other clathrate forming molecules very much involved in climate change, besides CF4, specifically propane, C3H8, and carbon dioxide. It also discusses the noble gas xenon, which as it turns out may play a role in nuclear reactors featuring continuous on line processing, but that's another story.
This paper is about clathrates. From the introduction:
It was proposed that the nature of this difference may be associated with crystal anharmonicity which is larger in hydrates than in ice.(5) Taking the crystal anharmonicity into account, the thermal expansion of both the empty and gas hydrate with cubic structure I (CH4 and Xe sI hydrates) and with cubic structure II (Ar, Kr, and C3H8 sII hydrates) were studied using the lattice dynamics approach.(6) It was shown that the thermal expansion coefficients of the hydrates depends on the size of the guest species. Thus, the value of the thermal expansion coefficient of the hydrate with large guests is smaller than that of hydrates with small guest molecules, with the empty lattice having the smallest value. Compression of the hydrate lattice was observed in the case of filling by small guests, argon, and krypton.
A few diffraction-based experimental studies of the dependence of the lattice parameters on thermal expansion have been performed recently, both on laboratory-made and natural CH4-hydrate samples at thermodynamic conditions of interest to geoscience and chemical engineering research, including temperatures close to 0 °C and ambient pressure.(7)
Neutron- and synchrotron diffraction experiments were used to determine the lattice constants of the CH4, CO2, Xe, and N2 clathrate hydrates in the temperature range from 10 K to the stability limit.(7) It was also found that the thermal expansion of gas hydrates is considerably larger than the thermal expansion of both hexagonal ice and the empty hydrate with cubic structure II.(8) This indicates that the guest molecules are affected on the thermodynamic behavior of hydrates. Moreover, the origin of the large deviations between different experimental data for thermal expansion reported in the literature(2,7) is unclear, as are the reasons for these deviations. The large scatter in previously published lattice constant data for the gas hydrates may be related to differences in experimental facilities and synthesis methods that impact sample quality and cage filling, and in the case of natural samples, the influence of impurities.(7) At the same time, the accuracy of the calculated values of the lattice constants depends on the selected model and interaction parameters.
It is important to note that this particular paper refers to clathrates in solid state water, more or less. However, it is important to note that any state of matter is actually a canonical ensemble of multiple microstates: In a sense, a state of matter is a statistical average of micro or nano states. At the extreme level, as in the statistical mechanical theory of gases, a state can be considered a statistical ensemble of gas molecules or atoms. It is true that even in hot water, there are microregions where, temporarily at least, the structure of water is similar to that found in ice; likewise in ice, there are regions that have the properties of liquid or even steam. Indeed, this explains why and how ice sublimes and exhibits a vapor pressure.
Henry's law is a law of physics that relates the solubility of a gas to the partial pressure of the gas above it, and is, simplified, a pure proportionality relationship between pressure of a gas over a liquid and its solubility in that liquid. We observe the effects of Henry's law every time we notice the outgassing of carbon dioxide in a carbonated liquid such as soda or beer or champagne when we pop the container open. Reducing the pressure of carbon dioxide above the liquid in the container to that of the atmospheric partial pressure, causes the beverage within which the carbon dioxide is dissolved to outgas.
Deviations from Henry's law in some gases, particularly over water, are often explained by appeal to clathrate formation. In general, clathrates in water can have different structures, defined by hydrogen bonding, generally common in two different structures: Type S1 and type S2, both of which exhibit cubic symmetry. Other clathrate structures exist. For a nice sophisticated overview of clathrate structures, see Prediction of Clathrate Structure Type and Guest Position by Molecular Mechanics (Fleisher and Janda, J. Phys. Chem. A 2013, 117, 4001?4010)
In consideration of these facts, it is justified to consider the solid state clathrates discussed in this paper in the context of fluid phases. Indeed, the formation of clathrates can induce state changes. This is a much discussed and studied phenomenon in pipelines for the transport and/or mining of dangerous natural gas.
However the purpose of this paper is not to discuss Henry's law deviations or solubility, but chiefly concerns the size associated with these clathrates on a molecular scale in the solid phase, that is, as a structure essentially in ice, not a liquid, although, again, these clathrate ices are in fact, statistical canonical ensembles.
A little physical chemistry porn addressing the size of clathrates:
 (1)
(1)
By determining the sum of the j-th frequency of crystal vibration, ?j(q⃗ ) at different wave vectors, q⃗, the vibrational contribution can be calculated using the following formula
 (2)
(2)
The summation over the frequencies in the interval [0??m] can be reduced to the integral
 (3)
(3)
where
 is the vibrational density of states (vDOS).
is the vibrational density of states (vDOS).
All frequencies ?j(q⃗ ) of the harmonic representation of the free energy in the quasiharmonic approximation depend on the volume of the system and should be calculated for every volume separately. Therefore, to obtain the free energy dependence on volume, calculation of the same system at different volumes is required.
At fixed temperature, the equation of state P(V) can be calculated using the following expression
 (4)
(4)
This equation allows us to find the volume of the system at the required pressure and temperature and thereby determine the temperature dependence of the absolute values of the structural parameters and thermal expansion of the hydrates. The molecular coordinates have been optimized by the conjugate gradient method prior to calculating the free energy. This method determines the new coordinates of the molecules from the minimum of the potential energy of the expanded lattice.
The interaction between water molecules in ice Ih and the clathrate hydrate phases has been described by the modified SPC/E (SPC/Emod) water–water interaction potential
 (5)
(5)
The Lennard-Jones (L-J) parameters describing short-range interactions are ? = 3.1556 Å; ? = 0.65063 kJ/mol. The charges on hydrogen and oxygen are equal to +0.4238 |e| and ?0.8476 |e|, respectively.(12?15) The 4×4×2 supercell with 128 water molecules was used for the calculation of ice Ih. The approximation of spherically symmetric L-J particles was applied for the guests with the interaction parameters presented in Table 1.
Equation (5) is a statement of the Lennard Jones potential, which measures how attractive forces at one interatomic distance become repulsive forces when molecules become to close. Sir John Lennard-Jones (1894-1954) was born John Jones, but in 1925, he married Kathleen Mary Lennard and added her name to his. That was very, very progressive given his times…
…My kind of guy, Professor Sir John Lennard Jones. He is considered a "father" of computational chemistry.
...Anyway...anyway...
Some graphics from the paper:

The caption:
The SPC/E model is a model of water called the "Single Point Charge/Extended" model. It, and related models of water are discussed here: Structure and Dynamics of the TIP3P, SPC, and SPC/E Water Models at 298 K (Pekka Mark and Lennart Nilsson, J. Phys. Chem. A 2001, 105, 43, 9954–9960)
Some text from the paper:
Figure 2: Vibrational density of states for clathrates:

The caption:
Figure 3:

The caption:
The most important clathrates on Earth right now, that we have bet the entire planet's geological history on reactionary fondness for so called "renewable energy" - this because in the minds of some people, Fukushima was a worse disaster than people dying in the streets during heat waves, the coasts of continents breaking into fire, six to seven million air pollution deaths per year, crop failures, droughts, the increase range of pathogenic vectoring insects, oh, and mining coal and iron to make steel for wind turbine posts - are the ice clathrates of carbon dioxide and methane:

The caption:
The reason that these clathrates are important is that these two major greenhouse gases are sequestered in permafrost, in ice clathrates, that are now melting because we did nothing but buy into Greenpeace horseshit about climate change (as if that contempt for science and engineering was enough). This is far more serious than navel gazing asses who lack a shred of scientific insight losing sleep at night about the release of weakly tritiated water being released into the sea at Fukushima. The melting of clathrates will kill people, because climate change is killing people, even as we wait for the grand so called "renewable energy" nirvana that never came, isn't here, and won't come.
And now the two clathrates, those of xenon and CF4, that drew my interest to this paper, owing to my strong interest in nuclear engineering, the chemistry of valuable used nuclear fuels and the use of radioactive materials to do what no other materials can do as easily, to permanently destroy intractable extremely long lived, high greenhouse gas potential, fluorinated gases, as well as N2O:

The caption:

Finally the clathrates of nitrogen and propane:
The caption:
So far as clathrates go, those discussed in this paper that are of most relevance to the environment are clearly methane and carbon dioxide. These exist in vast quantities both in permafrost, and in high pressure regions in deep oceans. As to the extent that van der Waals–Platteeuw theory, and violations of it, have on the release of these clathrates as the permafrost in arctic regions, they are irrelevant. These effects may speed things up a little but the reality is that, one way or another: The release of these greenhouse gases from clathrates is now inevitable. At the beginning of this century, we bet the entire planet on wind turbines and solar cells, a reactionary return to the belief - abandoned during the 19th century for damned good reasons - that so called "renewable energy" could and would sustain the world. So called "renewable energy" did not sustain the world; it isn't sustaining the world; and it won't sustain the world. This belief, like most pernicious myths, from the Trumpian delusion that he won the election to the precisely equivalent belief that someday we will mine enough cobalt, enough copper for a vast array of transmission lines, enough neodymium, to make ever more elaborate Rube Goldberg contraptions that will make what can't work, work, is destroying more and more of the future. These myths are not neutral; they are not without consequence; they kill people and they are killing the future.
The failure of the myth that so called "renewable energy" could sustain the world is written in the planetary atmosphere.
The data is unambiguous: The Data.
It's a fact. Facts matter.
For the week of May 13, 2018 the 2018 weekly average maximum for the dangerous fossil fuel waste carbon dioxide concentrations measured at the Mauna Loa observatory was 411.85 ppm, then an all time record. This record was surpassed during week of January 20, 2019, when the weekly reading was 411.99 ppm. The maximum for 2019 was reached during the week May 12, 2019, when the reading was 415.39 ppm. In 2020, well into the Covid crisis in China, the world's largest dumper of carbon dioxide, and the second largest per capita dumper after those of us in the "Green" United States, the 2019 weekly average maximum was passed on March 20, 2020, when the reading was 415.52 ppm. The 2020 weekly average maximum was reached for the week beginning May 24, 2020 was 417.43 ppm, the current all time record.
We will smash that record this winter or early spring. I suspect that the maximum for 2021 will either surpass or scrape 420 ppm.
We are doing nothing meaningful.
Just for fun, I made a rare venture into the E&E forum here - where unwarranted belief in so called "renewable energy" is still a sacred cow fart - to make a post, just for amusement, alluding to Trumpian scale delusion. It was called, Wow, I just learned we're in the "renewable energy" era.
I wrote:
I had no idea. I keep thinking that carbon dioxide concentrations are going up at a record pace, but here were are past the "dawn of the renewable energy" era that I've been hearing about my whole damned adult life, and I'm not young.
I always thought that the "renewable energy era" would mean the end of the climate change problem. I guess I'm missing something.
It's a new strategy, very popular in some circles I think: When you've lost and failed, claim you won and succeeded.
This is the Science forum, where facts matter.
It seems to have generated 14 responses, only one of which - by some person making money off the "renewable energy will save us myth - I was able to read by benefit of my use of the much appreciated and wonderful "ignore list." I have no more interest in listening to this kind of crap about how "renewable energy" will work some day or even worse, how it is working than I have in listening to Donald Trump, because to me, facts matter.
42°C is approximately 108°F. All over the world this summer, temperatures exceeded this temperature in many places around the world. The people who continue to write, in denial of reality, about how renewable energy will save the world are almost uniformly anti-nukes, the kind of people who worry more about a people eating a tuna fish containing a few atoms of Fukushima cesium than they do about tens of thousands of people dropping dead in heat waves. These people deserve no more attention than Trump. Their ignorance, like Trump's ignorance, kills people. Nuclear Power Learning and Deployment Rates; Disruption and Global Benefits Forgone
Again, what caught my eye about this paper, was the trace gas (and refrigerant) CF4 since I have considered a situation in which small amounts of it might be generated in a process about which I thought in which the reprocessing of valuable used nuclear fuels - most of which are oxide based fuels - using anodic dissolution in a rubidium based eutectic. (Rubidium is a fission product.) (Eutectic salts are widely used industrially, most notably in the aluminum industry, but will be likely to be used more widely for metal refining in the marvelous Cambridge FFC process.) My interest was drawn, since I am aware that CF4 in an aqueous phase will not be radiologically transformed into trifluoromethanol - which decomposes fairly rapidly - but to trifluoromethane, fluoroform, as discussed above, which is a worse climate gas than CF-4 itself.
Trace gases are now monitored by satellite, and will be for some time, at least until that asshole Elon Musk litters space with so much space debris the the use of satellites for any purpose will no longer be possible.
The concentration of fluorinated gases (other than the banned CFCs) is rising steadily: Sixteen-year trends in atmospheric trace gases from orbit. (Bernath et al., Journal of Quantitative Spectroscopy & Radiative Transfer 253 (2020) 107178)
Here is the trend and distribution of fluoroform (HFC-23):

Although fluoroform is a "heavier than air" gas, the apparent violation of the gravimetric distribution law is mostly likely a function of the fact that the gas is formed there from the radiative destruction not only of CF4, but other HFC refrigerants as well.
While these gases are measured in parts per trillion, it is important to recognize that the destruction of one kg of CF4 is the equivalent, for the purposes of climate - weighing global warming potential - to destroying 6500 kg of carbon dioxide. In the case of fluoroform, the destruction of 1 kg of fluoroform is the equivalent to the destruction of 50,000 kg of carbon dioxide.
We know that these gases are decomposing in the upper atmosphere's radiation field, because the concentrations, because the concentrations of the highly reactive (and toxic) but happily transitory molecule carbonyl fluoride (fluorophosgene), which was not included in the paper just linked, are detectable:
Satellite observations of stratospheric carbonyl fluoride (Harrison et al., Atmos. Chem. Phys., 14, 11915–11933, 2014). Fluorophosgene is readily decomposed to CO2 and HF by water. HF is readily neutralized by calcium carbonate and/or other bases.
Fluoroform, while it degrades more slowly than CF4 owing to the presence of the weaker C-H bond as compared to the three C-F bonds, does degrade in the gas phase, either by combination of the CF3٠ radical with the OH٠ radical to give unstable trifluoromethanol, or by molecular cleavage to give HF and the CF2 carbene: Communication: A hydrogen-bonded difluorocarbene complex: Ab initio and matrix isolation study Sosulin et al., J. Chem. Phys. 147, 131102 (2017)) An extremely interesting fact about the CF2 carbene is that both xenon and krypton can insert into it to give compounds with rare xenon carbon and krypton carbon bonds: [link:http://|Carbene-insertion noble gas compounds: FKrCF and FXeCF] (Sosulin et al., Chemical Physics Letters 744 (2020) 137211).
At one time here I was writing a long post about xenon, a fission product, as well as it's volatile periodic table neighbors, tellurium, iodine and cesium, with an eye to improving their high value by in line isotopic separations by exploiting the variable of time. (I never got around to finishing it or posting it.)
Flouroform can also be decomposed in the gas phase to give carbon metal triple bonds (with three of the fluorine atoms bonded to the metal, and one fluorine bonded to the carbon. Here is one example of a paper written on this topic: Formation of unprecedented actinide carbon triple bonds in uranium methylidyne molecules (Andrews et al., PNAS November 27, 2007 104 (48) 18919-18924). This reaction is know to take place with other metals, including titanium, zirconium, hafnium and thorium.
Nuclear energy is often thought to apply to the generation of that famous and widely used, albeit thermodynamically degraded form of energy, electricity. However, high thermodynamic efficiency can be realized by treating nuclear energy merely as a source of clean and sustainable heat, with electricity generated as a side product and then only when needed.
In recent years, as my wasted life draws to a close, I have thought a lot about the potential of designs of nuclear reactors where Brayton type cycles are utilized with air as a working fluid, just as air is widely utilized as the working fluid in jet (Brayton) engines. In these Brayton cycles, in jet engines, air quality is degraded. It occurs to me though that it is possible to imagine Brayton cycles where the air is cleaned, where CF4, among other components, can be decomposed in the gas phase, without the generation of much fluoroform, indeed with the destruction of fluoroform. We have learned quite a bit about the behavior of fission products from the disasters at Fukushima and Chernobyl - those events which anti-nukes consider more important then the total destruction of the planetary atmosphere, acidification of the oceans, the total destruction of vast marine and terrestrial ecosystems, the coasts of continents in flames, the deaths of tens of millions of people every decade from air pollution, and now, people dropping dead from heat, droughts, and the destruction of the glacial sources of water, notably in Asia and in Europe... etc., etc.
This information that we have gathered from these events, along with the forensic materials science analysis of used nuclear fuels over more than half a century suggests to me that it may be possible to apply this knowledge irradiate the atmosphere on a very grand scale, that is to bring the stratospheric and ionospheric chemistry down here to the troposphere, this to reverse the vast damage done by fear, ignorance, and healthy dollops of denial, indifference, selfishness and sheer stupidity.
Over the last few weeks, I have not allowed myself the pleasure of writing a pure science post in the science forum of this political website. Most of my posts here on this political website have been (gasp) political. I learn a great deal by writing these posts, which is why I write them and I am very happy to have felt enough ease to write one again.
I am feeling more optimistic now that the orange nightmare is about to be consigned to the dregs of history, but with a caveat. It is not enough to defeat a transitory political evil, a pernicious ideology: It is more important to govern well and to develop an ideology that will do good. While we mock the delusions of the right, we are not immune to pernicious delusions of our own. Our energy dogma, while correct in recognizing that the climate crisis is very real, is largely and vastly insufficient in recognizing what what must be done about. Let me be blunt: Wind turbines and solar cells will not cut it. They are distractions, wasteful distractions.
The last time the United States nearly fell apart, Abraham Lincoln implored the citizens of his time to "think anew." In this near event, these times, with the United States strongly at risk of falling apart, we would do well to take this general advice again. The climate crisis is more important and will last longer and be far more difficult to address than was the crisis of having an ignorant corrupt racist in the White House, far more important than having racist middle American hayseeds granted inordinate power in government. It is not enough to govern for all Americans, we must govern for all humanity, and for all the future.
If we truly think anew, among other things, we will recognize a basic fact - and it is a fact: Opposing nuclear energy is a crime against humanity. We seem to be having a hard time recognizing it, but it is a fact all the same, shown experimentally, with data collected over more than half a century. This may seem strong; it may seem harsh, but it is a fact. Facts matter.
I am very impressed with President Elect Biden and Vice President Elect Harris, but even as they glow and even as we bask in that hard won glow, we face a greater darkness, especially if we do not "think anew." They are not Gods; they are human beings. They cannot do it alone; we are all involved. The task is not over; it is just beginning. Let us help them. Let us think anew.
I trust you are enjoying a safe and reasonably pleasant weekend.
'It's like politicizing toilet paper.' A member of Biden's COVID-19 panel surveys the task ahead
Here's an interview with Dr. Céline Gounder, a physician and epidemiologist at NYU, who is on President Elect Biden's taks force. It's an item in the current issue of Science, a publication of the American Association for the Advancement of Science:
‘It’s like politicizing toilet paper.’ A member of Biden’s COVID-19 panel surveys the task ahead (Warren Caldwell, Science, November 13, 2020). It is most likely open sourced.
Some excerpts:
A: I have a lot of experience in terms of public health and clinical medicine when it comes to epidemics.
I led the Bureau of Tuberculosis (TB) Control in New York City, another respiratory infection. Contact tracing historically has been based out of TB. I worked in sub-Saharan Africa from the late ’90s, up until 2012, on tuberculosis and HIV, public health programs, and research. Then I was also an Ebola aid worker in Guinea.
Q: You also have quite a presence in the communication world. You have a podcast. You’re working on a movie. You’ve written articles for a variety of publications. Do you think that plays a part in it?
A: I’m sure. A big part of public health is communication. How do you convince people to wear a mask? How do you message around social distancing?...
...Q: So how do you get people to wear masks?
A: It’s a challenging one, because the issue has been so politicized. But in my mind, it’s like politicizing toilet paper. It’s a basic hygienic measure. It’s not about your political party. And we need to somehow break through that.
I will say people are doing better. If you look at the surveys, people are not perfect about wearing masks, but they are wearing them more. Part of this is also just letting people know that this is becoming the social norm. People are more likely to do something if they feel like everybody else is doing it...
...Q: Has the Trump administration’s unwillingness to acknowledge that Biden is the president-elect affected the task force’s ability to get its work done?
A: It’s certainly not an ideal situation. But you have many people, including Biden himself, who have very long careers working in government. So while you may not have cooperation occurring in any official transition capacity, it’s not like they’re in the cold.
There’s also nothing to prevent the transition team from interfacing with governors and state and local health officials. Finally, much of health care is delivered in this country by the private sector. In particular with respect to tests and vaccines, and monoclonal antibodies, that’s really about collaborating with the private sector to figure out how to scale up and distribute...
The full interview is worth a read.
Dr. Gounder:
 ?itok=xFNn37WW
?itok=xFNn37WW
What this is all about...
The fragile weak ego racist knows he's going to jail because, while he knows very little, he knows he's a criminal.
He's panicking and grasping at straws.
Straws will not float one in a hurricane.
Wow, I just learned we're in the "renewable energy" era.
I came across a paper today in a journal I read regularly, Enabling Superior Electrochemical Performance of Lithium-Rich Li1.2Ni0.2Mn0.6O2 Cathode Materials by Surface Integration (Guo et al., Ind. Eng. Chem. Res. 2020, 59, 43, 19312–19321) which begins like this:
Wow.
I had no idea. I keep thinking that carbon dioxide concentrations are going up at a record pace, but here were are past the "dawn of the renewable energy" era that I've been hearing about my whole damned adult life, and I'm not young.
I always thought that the "renewable energy era" would mean the end of the climate change problem. I guess I'm missing something.
It's a new strategy, very popular in some circles I think: When you've lost and failed, claim you won and succeeded.
Nice cathode, though, no cobalt, which should reduce the reliance on the child slaves who dig cobalt in the Congo River region.
Would anybody here like to see Adam Schiff appointed to VP-elect Harris' Senate Seat?
Just asking.
By my calculation off the CNN vote tallies, Trump would need 57.6% of the remaining vote to tie GA.
With 99% of the vote reportedly in, from the total votes cast, 2462099 for Biden and 2454552 for the corrupt orange racist, equaling a total of 4916651 between the two, and 4916651/0.99 = 4966314, and 4966314/2 = 2483157 (tie), Trump would need 28605 of the remaining votes, and Biden only 21058.
28605/(28605+21058) = 0.576.
These figures ignore third party candidates, and in actuality there are only two significant figures (0.99).
Unless the remaining ballots are in very red areas, it ain't gonna happen.
Trump Defends Lawsuits: "No One Knows More About Fraud Than Me"
Trump Defends Lawsuits: “No One Knows More About Fraud Than Me”WASHINGTON (The Borowitz Report)—Donald J. Trump offered a full-throated defense of his election-related lawsuits on Thursday, arguing, “No one knows more about fraud than me.”
Trump ridiculed television commentators who have dismissed his accusations of election fraud, claiming that he has “much more experience in fraud than all of these beauties put together.”
“People forget that, right when I became President, I settled a twenty-five-million-dollar fraud case against me,” he said. “You can’t beat hands-on experience like that.”
“For my entire life, I have been drenched in fraud,” he said.
Trump said that, when his election lawsuits are argued in court, his special expertise will win the day. “Fraud is my middle name,” he boasted.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,516