NNadir
NNadir's JournalElectrolysis of Lithium-Free Molten Carbonates
The paper I'll discuss in this post is this one: Electrolysis of Lithium-Free Molten Carbonates (Xiang Chen, Zhuqing Zhao, Jiakang Qu, Beilei Zhang, Xueyong Ding, Yunfeng Geng, Hongwei Xie, Dihua Wang, and Huayi Yin ACS Sustainable Chemistry & Engineering 2021 9 (11), 4167-4174)
I've argued before in this space that electricity is a thermodynamically degraded form of energy: Synthesizing Clean Transportation Fuels from CO2 Will at Least Quintuple the Demand for Electricity.
I have also argued, sometimes while addressing the stupid but oft discussed solar thermal energy fantasy, that the thermochemical splitting of carbon dioxide into carbon monoxide - from which pretty much any industrially produced carbon compound can be made (just add water) - and oxygen is the most efficient approach to carbon dioxide reduction: Cerium Requirements to Split One Billion Tons of Carbon Dioxide, the Nuclear v Solar Thermal cases.
Although for certain reasons I am very fond of the cerium based thermochemical splitting of carbon dioxide, many other such catalytic systems are known for this highly endothermic reaction.
The international symbol for dangerous fossil fuel free energy has become a wind turbine or solar cell graphic object, which is frankly as silly as waving a graphic of a Roman executioner's cross as a symbol for the way to address immorality; both are faith based. It has been experimentally verified, at a cost of trillions of dollars, that wind turbines and solar cells are not even remotely capable of addressing climate change. In fact, the worship of them - and let's be clear that it's nothing more than faith based worship - has led to the acceleration of climate change. The average concentration of the dangerous fossil fuel waste carbon dioxide in the atmosphere measured at the Mauna Loa Carbon Dioxide Observatory during the week beginning on March 19, 2000 was 370.98 ppm. This morning, the latest figure was 417.67 ppm. The current 12 month running average of weekly measured increases over carbon dioxide increases over the last ten years is 24.24 ppm, 2.42 ppm/year. The same 12 moth running average for the week beginning March 19, 2000 was 15.14 ppm, 1.51 ppm/year.
These are facts. Facts matter.
Heckuva job humanity at addressing climate change with all those wind turbines, solar cells and electric cars, heckuva job.
The graphic attached to the introduction of this paper is nonetheless the equivalent of the Roman execution's cross as applied to so called "green" energy. Here it is:

What I like about this paper is that it is cognizant of the fact that matter, specifically the individual elements in the periodic table, is not "renewable." The claim that we can save the world with batteries is no different than the claim that supplies of dangerous petroleum, dangerous natural gas, and dangerous coal are infinite, as well as the claim that places to put the waste, chiefly, but hardly limited to, carbon dioxide is unlimited.
They start out talking about lithium and then consider elements that are far more available, one of which, strontium, catches my eye. Overall this discussion of batteries clearly has some relevance to the "batteries will save us" fantasy.
From the introduction to the paper:
They have a nice graphic on the point:
The caption:
They continue:
The use of molten calcium salts is the essence of the FFC Cambridge process for the electrochemical reduction of metals, which I personally believe should be world changing, even if it is true that electricity is a thermodynamically degraded form of energy.
In saying this, they do raise some issues to be addressed:
These factors are what they examine in the paper, and then propose an electrode to reduce carbon dioxide to carbon, in effect reversing the combustion of the dangerous fossil fuel coal.
This process, which requires the input of a thermodynamically degraded form of energy, electricity, as well as heat suggests an important point that is often over looked, which is this: In order to capture carbon dioxide in air one needs to put more energy into the system than all of the original energy ever released by the original combustion of the dangerous fossil fuel produced. This reality should sober up all the drunken handwaving and wishful thinking that goes on when energy and the environment is discussed, but I confidently predict, on the basis of being a tired old man, that it won't.
Their important concern is thermodynamics:
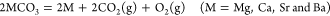 (1)
(1)
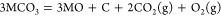 (2)
(2)
If the MO in eq 2 is soluble, then the carbonization reaction between MO and CO2 spontaneously occurs and forms fresh carbonate to maintain the concentration of CO32– of the electrolyte:
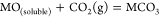 (3)
(3)
The overall reaction of eqs 2 and 3 is
 (4)
(4)
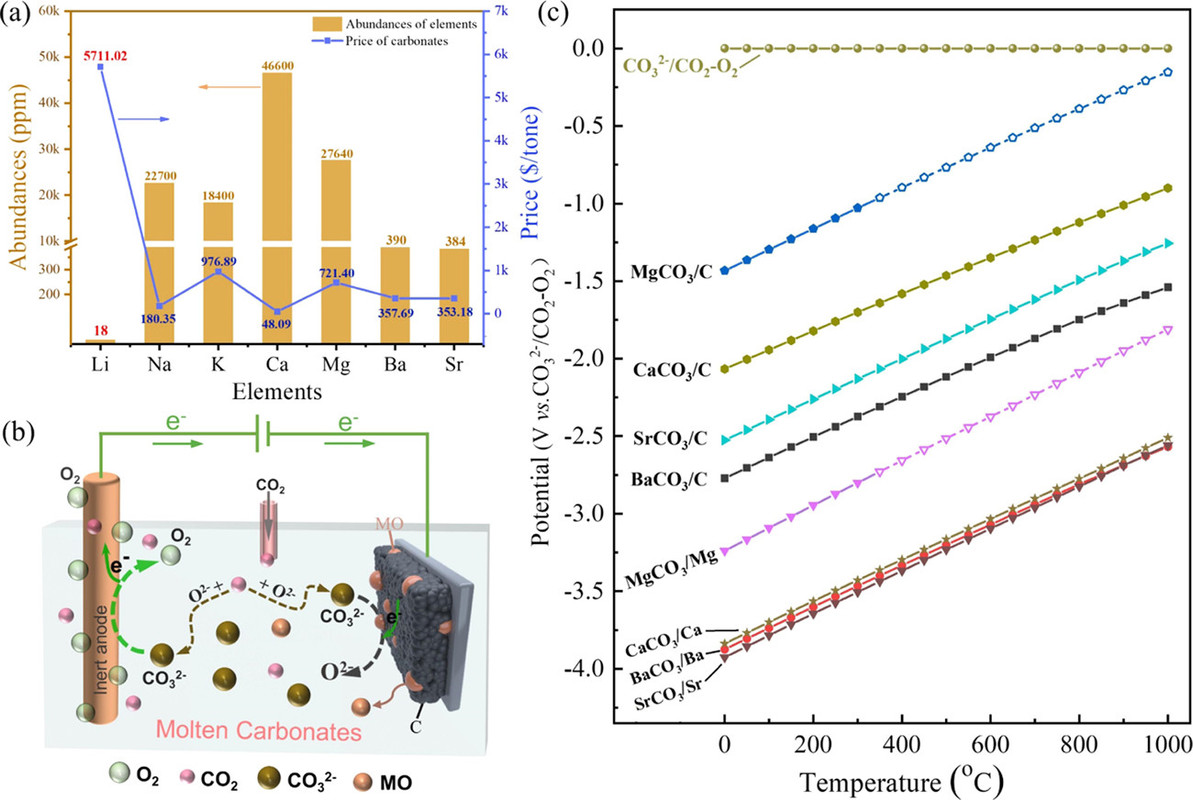
Thermodynamically, alkaline-earth metal carbonates (MCO3, M = Mg, Ca, Sr, and Ba) can be electrochemically converted to carbon at the potential prior to the deposition of the alkaline-earth metal (Figure 1c). Therefore, the deposition of carbon is thermodynamically easier than that of metal. Moreover, the thermodynamic favorability of generating carbon is different depending on the different alkaline-earth metal cations. For example, the thermodynamic deposition potential sequence follows CaCO3 < SrCO3 < BaCO3. In other words, CaCO3 can be reduced to carbon at the potential more positive than that of SrCO3 and BaCO3. Note that the potentials of carbon generation in Na2CO3 and K2CO3 are more negative than that of the deposition of the corresponding alkali metals. Thus, Na2CO3 and K2CO3 are usually employed as the supporting electrolyte.(39,44) Therefore, alkaline-earth carbonates are the solute to be reduced for the carbon generation in MCO3–Na2CO3–K2CO3 (M = Mg, Ca, Sr, and Ba).
The reduction behaviors at a Mo electrode in a variety of carbonates containing different alkaline-earth metals are studied. As shown in Figure 2a, no reduction peaks were observed before the cathodic limit in the pure molten Na2CO3–K2CO3, demonstrating that Mo is an inert material that does not involve in any electrochemical reactions in the selective potential range.
The preliminary experiments investigated the cyclic voltammograms of molten carbonate systems using molybdenum electrodes:
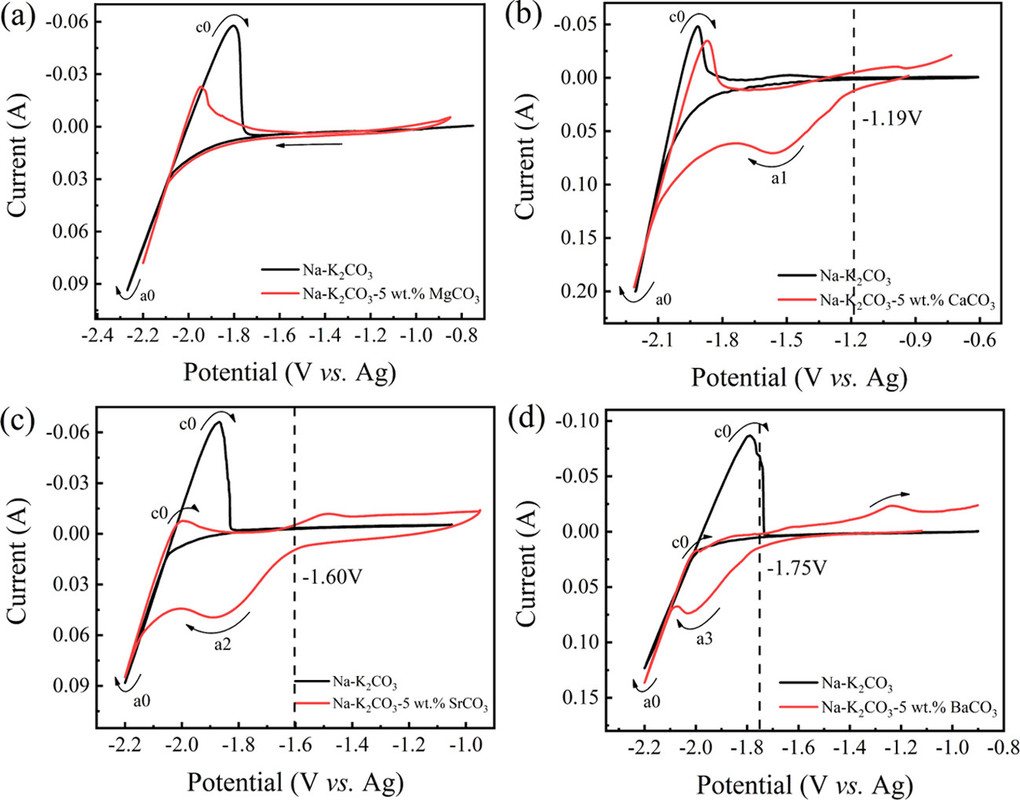
The caption:
Carbon was indeed deposited under these conditions, at a temperature of 750°C in sodium/potassium carbonate melts containing 10% (by weight, surprisingly) of three alkali metal carbonates, those of calcium (as in the FFC Cambridge Process), strontium and barium.
Micrographs of the carbons formed are shown:
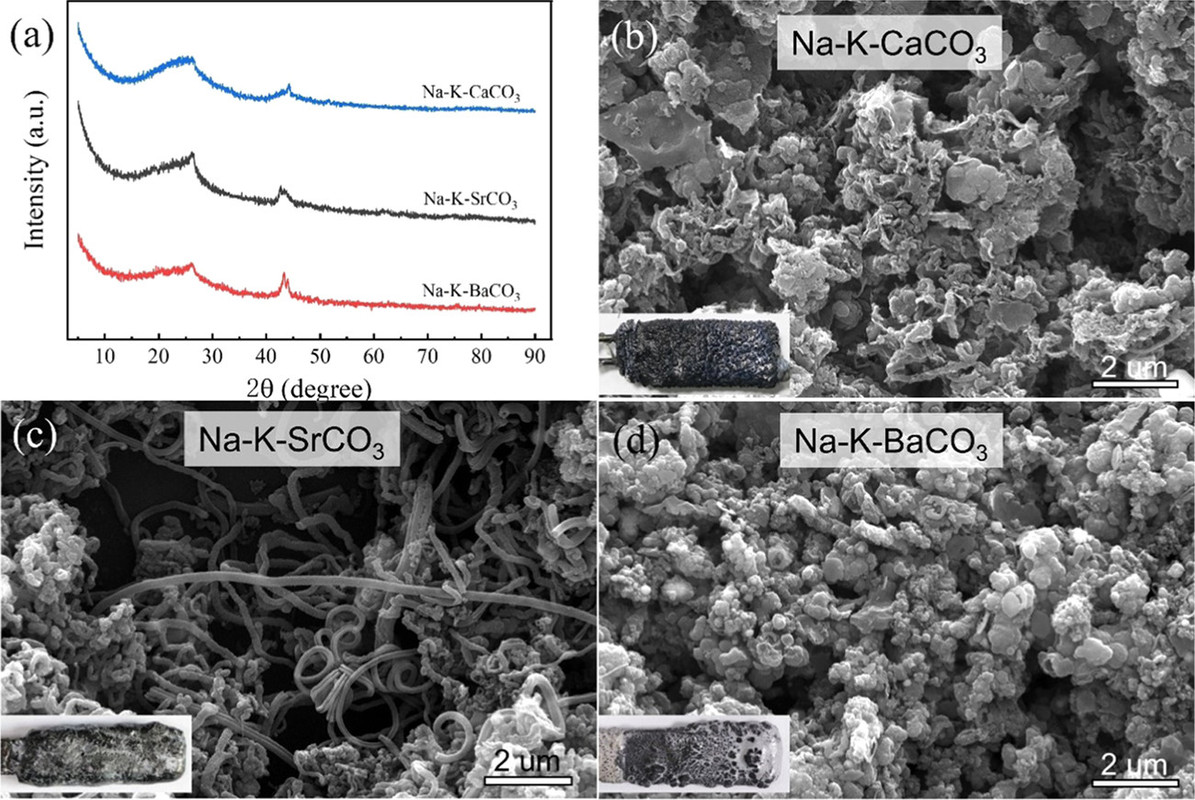
The caption:
The question next turned to finding a stable electrode for the oxidation side of the reaction:
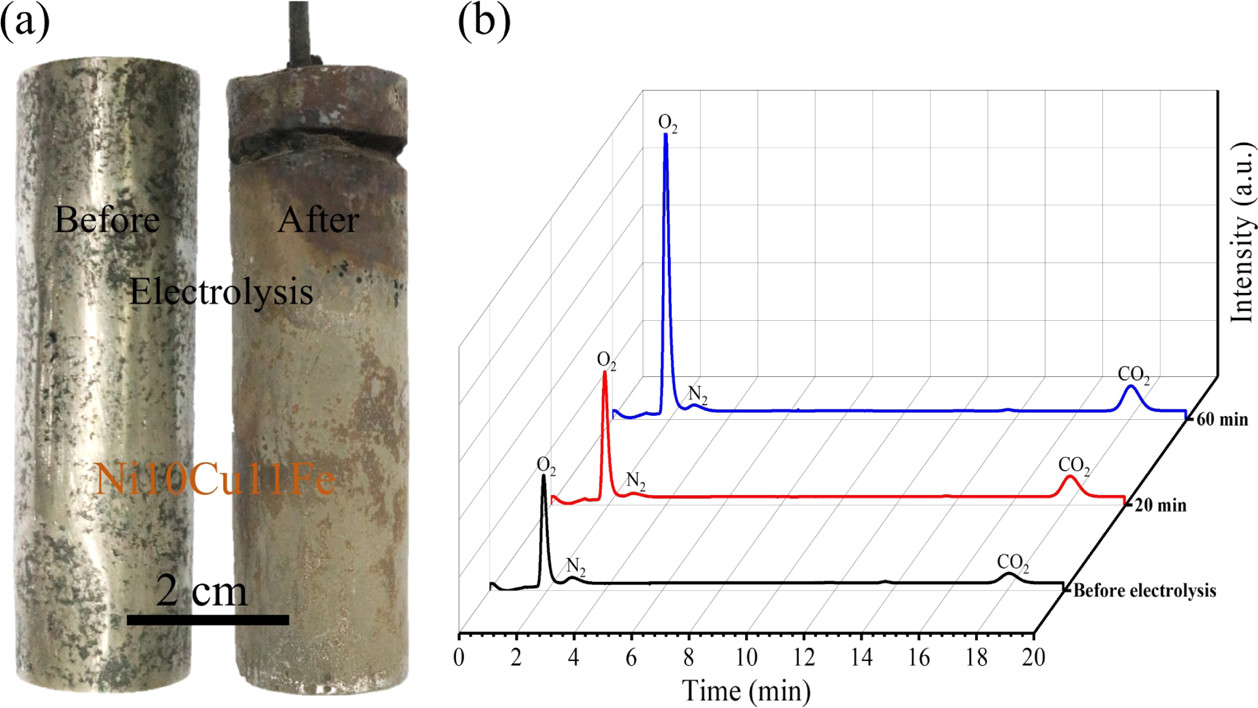
The caption:
Next the solubility of the various oxides, calcium, strontium, and barium were determined by the simple expedient of putting pellets of these oxides in the melts and observing the amount dissolved:
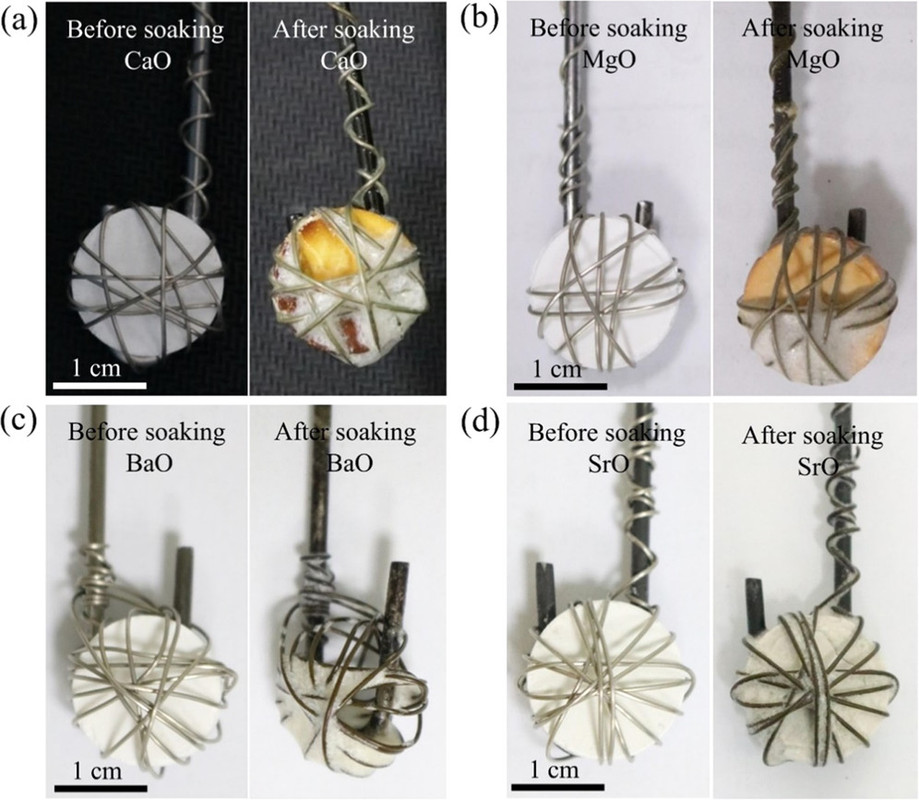
The caption:
Quantitative tables of the solubility of these oxides are not given in the paper, but the general conclusion was that barium oxide was the most soluble and the solubility of its oxide and carbonate were evaluated in more detail along with the rate of dissolution, as shown in the following graphic:
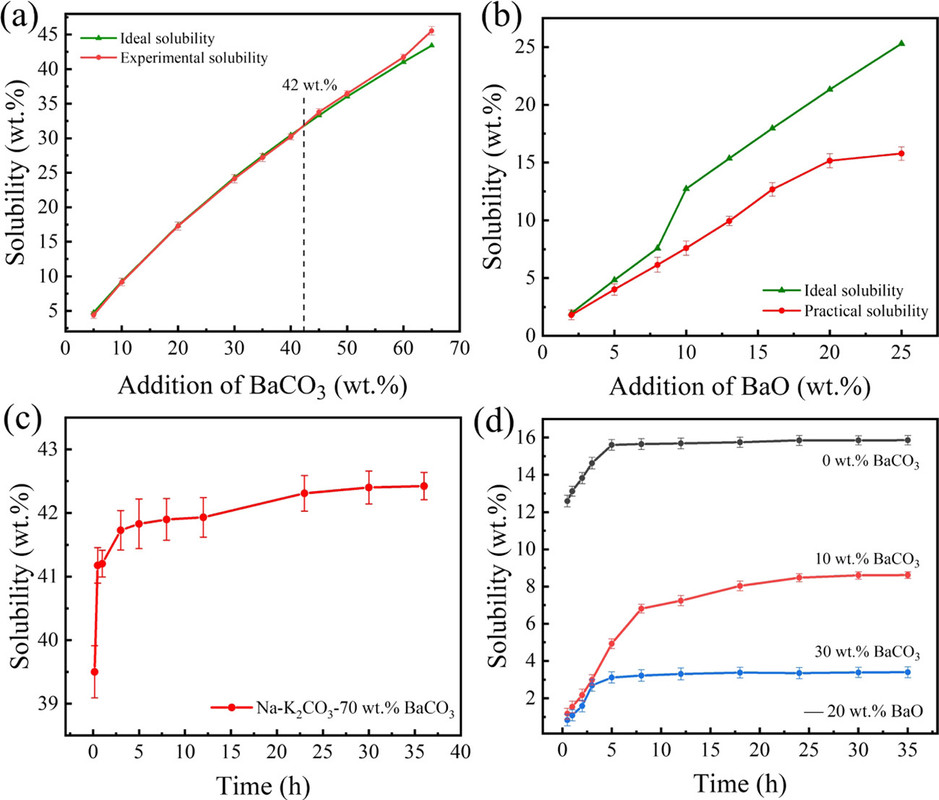
The caption:
It would be nice, from my perspective, if a more complex carbonate system allowing for the use of strontium in this system were considered in subsequent optimization, since strontium is available from used nuclear fuel with a heat generating isotope, Sr-90, which might defray the environmental and economic cost of maintaining a melt, although there are many heat network setting which might address this problem at an acceptable environmental and economic cost.
In any case, the barium oxide can absorb gaseous oxygen, even at these high temperatures - the decomposition temperate of barium carbonate is around 1300°C.
If the goal is, however, to produce the oxides themselves of strontium and/or calcium - the latter oxide is a key constituent of concrete, concrete production being a major contributor to climate change, the insolubility of the oxides is actually a desirable outcome.
The authors write:
This figure obviates that point:
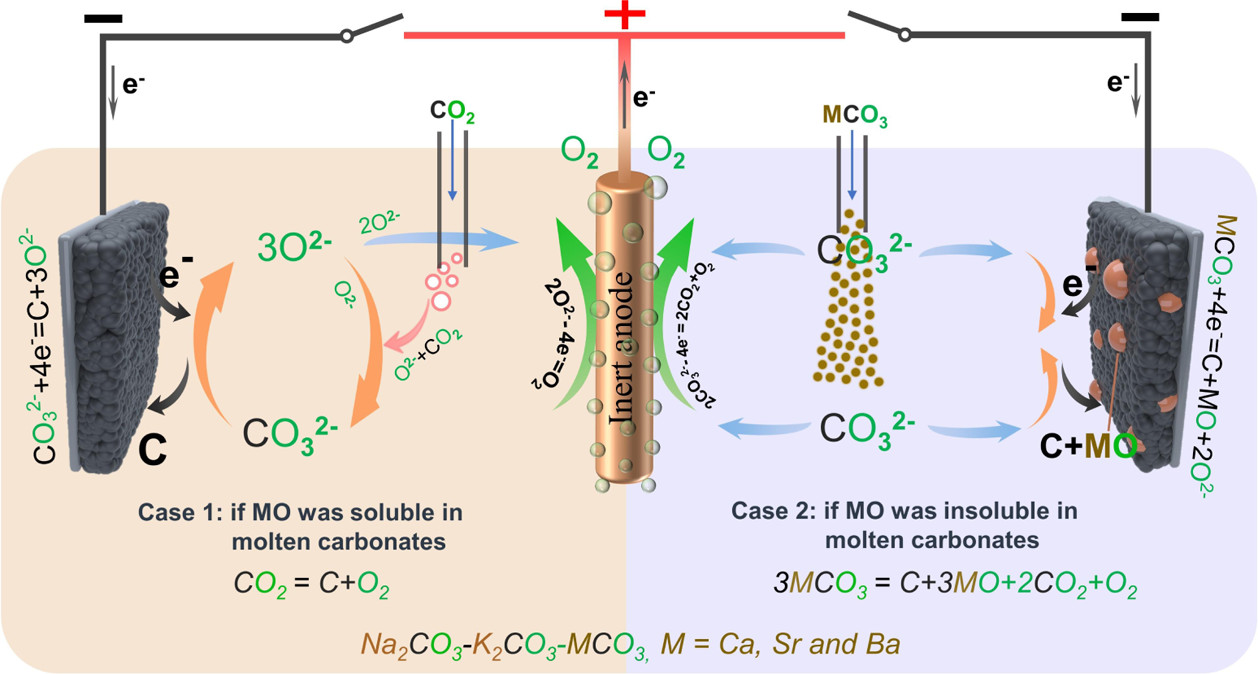
The caption:
They then write about capturing carbon dioxide from flue gas; if this gas is formed to generate electricity, they are then talking about a perpetual motion machine, but that doesn't totally negate the value of the paper itself.
I began this commentary by noting that electricity is a thermally degraded form of energy, but did not state the caveat that electricity captured by use of waste heat that would otherwise be rejected to the atmosphere is merely an improvement in overall energy efficiency. In the case where electricity is generated as a side product, using waste heat from a very high temperature process, the second law of thermodynamics is still operative - there is no physical way to make it inoperative - and increased exergy is obtained.
The conceit surrounding the so called "renewable energy" fantasy is that the intermittent nature of electricity can be addressed by net metering. On the left, we like to mock the statements of the assholes running ERCOT and the racist governor of Texas that the recent events in that State that led Senator Cancun Cruz to run away, the collapse of its power system was an outgrown of the so called "Green New Deal." To be perfectly clear and unambiguous, even as a lifelong Democrat, I don't think that the "Green New Deal" is going to be green or much of a deal. I have zero respect for the tired and old, and frankly experimentally failed energy ideas of Ed Markey, whether I otherwise agree with his other politics, as I often do. So called "renewable energy" is failing us now, and worse, is failing the future. It didn't work to address climate change. It isn't working to address climate change. It won't work to address climate change. Period. A key concept in the ideology of Green New Dealism is so called "net metering" which is the idea that the use of electricity follows its availability, its availability determining its price. If we are honest with ourselves, not that it is easy for anyone to be honest with themselves when honest confronts faith, including quasi religious faith, even absurd faiths, the extreme utility bills seen by some citizens of Texas are "net metering" run wild.
A heat network run by a high temperature nuclear plant, one producing temperatures high enough thermochemically split carbon dioxide - the cerium cycle running at maximum temperatures of 1400°C - will achieve the highest efficiency - the yield of exergy - as a constituent of a heat network, possibly producing a number of Brayton, Rankine and even Sterling cycles in sequence. If this is the case, electricity may be inevitably a side product. If this electricity is used to run combustion in reverse, which is what this paper is all about, the electricity can be switched to the grid whenever electricity prices rise high enough to justify the switching. In effect, a chemical plant is run as spinning reserve. This is not a new idea. Kaiser aluminum - aluminum production is an chemoelectric process - did this historically with hydro power in the Pacific Northwest.
Power demand on the grid fluctuates regularly, with the highest demand in the late afternoon and early evening, precisely when the sun goes down ironically enough, since so many people actually believe that solar electricity will save the world. It won't. It hasn't. Exploiting these fluctuations can be utilized to produce molecular carbon from CO2 gas or provide electricity to the grid, depending entirely on the value to the operator of the plant.
From the paper's conclusion:
It's a very nice paper. I like it a lot.
I trust you are enjoying your weekend.
Use of a Modified SIRD Model to Analyze COVID-19 Data
All of the scientific publishers have made all Covid-19 related papers open sourced, so there is no need to discuss this paper in any detail as interested parties can read it themselves, but here it is: Use of a Modified SIRD Model to Analyze COVID-19 Data (Devosmita Sen and Debasis Sen Industrial & Engineering Chemistry Research 2021 60 (11), 4251-4260.)
Here, anyway, is an excerpt including the definition of "SIRD:"
ARTICLE SECTIONSJump To
Since the beginning of 2020, the whole world has been experiencing a major and unprecedented global crisis, owing to the outbreak of the COVID-19 pandemic.(1?3) The infection is resulting in severe, and sometimes even fatal, respiratory diseases such as acute respiratory distress syndrome.(4) Such an infectious disease, with a humongous social and economic impact was never seen before, at least in the recent past.(5) COVID-19 arose due to a strain of a novel coronavirus that has rapidly spread throughout the globe,(1,6) originating from and infecting a large number of people in Wuhan, China.(7?9) The spread of this disease has a complex time dependence, which is governed not by the number of infected people alone, but is strongly correlated with aspects such as total population of the country, various norms and measures taken by the nation at a particular time, and many more.(10?14) Because of a lack of previous experience in controlling a similar pandemic with such a high impact in the recent past, it is difficult to anticipate the size of the population that may get affected by this pandemic and the typical time required for its control.
The abovementioned crisis immediately calls for a quantitative understanding of the time evolution of this complex and non-linear process through computer modeling. Statistical and mathematical analysis of reported data can provide valuable insights into the trend of the spread and thus can assist in planning various social measures to contain the spread of the virus as quickly as possible. Further, analysis of reported epidemic data plays a vital role in analyzing the underlying phenomena involved in spreading of the disease and to make predictions about future trends. This enables various organizations to efficiently plan their steps toward containing this spread.
In literature, a few models have been proposed to explain such data. These can primarily be classified into two categories, collective models(12,13,15?18) and networked models.(19?23) Some examples of the former class of models are growth and logistic models,(12) the susceptible–infected–recovered–dead (SIRD) model, and their modifications,(9,16) collectively termed as compartmental models. At this juncture, it is worthy to mention that such a phenomenon has a close resemblance to kinetics of chemical reactions in general,(24) where transition from one state to another is associated with a specific rate. In epidemic modeling, the corresponding rates may be expressed in terms of the instantaneous number of infected, recovered people, and so forth. Owing to the complex interdependence of several processes governing the spread of infections and recovery, a proper mathematical model should be able to simultaneously predict the temporal behavior of infected, recovered, and dead people. As the protection procedures demand quarantine, confinement, social distancing lockdown measures, and so forth,(26) a simple SIRD model only gives a preliminary understanding of the process and is not sufficient to describe such complex processes in general. Here, we model the spread of the present pandemic using a generalized SIRD model, taking into account the fraction of population which is exposed, under quarantine, confined, active-infected, recovered, and expired at time instant t. It is noteworthy that proper modeling calls for a simultaneous corroboration of the time evolution of all the three independent reported data sets, namely, active number of infections and cumulative number of recovered and expired people due to the infection.
In this manuscript, we report the analysis of COVID-19 time series data for five countries—China, Italy, France, the United States, and India (and two of its highly affected states)—using the modified SIRD model. We have shown that this model is capable of explaining the current data significantly well for all these five countries. It should be noted that the present approach is unique because it is capable of explaining simultaneously all the three reported data sets: active, recovered, and dead population. The data have been obtained from https://data.humdata.org/dataset/novel-coronavirus-2019-ncov-cases which is compiled by the Johns Hopkins University Center for Systems Science and Engineering (JHU CCSE) from various sources including the World Health Organization (WHO) and from reported data on Indian state websites https://arogya.maharashtra.gov.in/1175/Novel--Corona-Virus, https://gujcovid19.gujarat.gov.in/.
A picture:

The caption:
Enjoy if interested.
An obscure fact I never knew: All of the lanthanides except promethium exhibit a divalent state.
I was wandering around the scientific literature today and came across this very cool fact:
I added the bold.
Trimethylsilyl versus Bis(trimethylsilyl) Substitution in Tris(cyclopentadienyl) Complexes of La, Ce, and Pr: Comparison of Structure, Magnetic Properties, and Reactivity (Chad T. Palumbo, Lucy E. Darago, Cory J. Windorff, Joseph W. Ziller, and William J. Evans, Organometallics 2018 37 (6), 900-905)

Most surprising in this equation is the fact that this holds true for three actinides, thorium, uranium and plutonium. Weird.
I'm glad I found that out before I died.
I was going to plant my summer vegetables and herbs seeds this weekend, but...
...we're having a midweek freeze here in central New Jersey.
That sucks. It's beautiful weather today.
Beto O'Rourke: Leadership on the Front Lines
This is a virtual online event. If you sign up and are unable to attend at the time it runs, they usually send you a recording.
Sign up here for the March 29 event: Tuck Life Long Learning.
One scientist showing appreciation of another in the comments section of a journal.
We often don't think of the social aspects of a scientific life, but in fact, all human strengths and flaws, particularly those involved simply with personality are there. It is a pleasure to work with most scientists, but it is also true that some are oppressive cranks with a nasty edge, including, to be sure, arrogance, contempt and, in many cases, a sense of malignant superiority and dismissiveness.
Of course, most people can display these flaws from time to time; I'm hardly immune myself.
At the end of most issues of scientific journals there'll often be short section in which comments on previous papers are offered. This is particularly true of ACS Journals, which tend to dominate my reading.
Often these comments are critical of the published paper under discussion, sometimes highly critical, even to the point of barely disguised hostility, even contempt.
I was very pleased to see one today that praised a paper and then simply asked for more information.
It's here:
Comment: The Novelty of a Two-Step Aromatization Process (Pulkit Bajwa, Industrial & Engineering Chemistry Research 2021 60 (10), 4189-4190)
An excerpt:
...A design of experiments strategy was briefly mentioned in the beginning of section 3.2 of this article. Statistical analysis was not provided in the Supporting Information. Variations in reaction conditions may significantly change the outcome of the reaction. As explained in the other study(2) interactions between factors generally provide additional important information. Was this true for this two-step study? Statistical details such as main effects, interactions, or contour plots could reveal more information on this topic, particularly the second step. Therefore, I would like to ask for more information on statistical analysis and answers to questions that are asked above. Researchers would be interested in this information, which will certainly add value to the current study. Perhaps it would encourage engineers and scientists to conduct more detailed studies on aromatization of hydrocarbons from renewable sources and coke characterization, and use statistical data for optimization.
"Design of Experiments," often abbreviated as "DOE" is a statistical approach to evaluating parameters in a process to understand how these parameters interact to delineate a path to a desired outcome.
After these kind remarks, the authors of the paper under discussion replied:
Reply to “The Novelty of a Two-Step Aromatization Process” (Swapnil Fegade, Brian Tande, Alena Kubátová, Wayne Seames, and Evguenii Kozliak Industrial & Engineering Chemistry Research 2021 60 (10), 4191-4191)
Further information about the statistical work performed during the study is available in Fegade(1) appendix C which contains all of the raw statistical data generated as well as additional statistical results from the Minitab program. These details were not included in the paper due to publication space limitations. Additional details can also be found in a patent by Seames and Tande.(2)
I can certainly appreciate when scientists "greatly appreciate" each other and be helpful to one another.
Science is a very human activity, and it's a joy when science can include graciousness. This is not always possible, but when it is, and is exercised, I certainly applaud it.
A trade-off between plant and soil carbon storage under elevated CO2
The paper I'll discuss in this post is this one: Terrer, C., Phillips, R.P., Hungate, B.A. et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 591, 599–603 (2021).
The abstract is open sourced, but it is useful to excerpt here to get some abbreviations understood:
It currently seems likely to me that this Sunday, a new weekly average record for concentrations of the dangerous fossil fuel waste carbon dioxide will be set at the Mauna Loa CO2 observatory, probably near, at, or above 418 ppm. (The previous all time record was established in the week beginning February 28 of this year, 417.97 ppm.)
Last year, for the week beginning March 22, 2020, the weekly average reading at Mauna Loa was 415.52. The high for the year, which established a new record until this year, was 417.43, 1.89 ppm higher, observed for the week beginning May 24, 2020. All this suggests that we may see a weekly average reading of close to 420 ppm this year, possibly above it.
We are doing nothing to address climate change. Nothing beyond reciting the usual myths on our side of the political spectrum (so called "renewable energy" will save us) or simply engaging in lying and denial on the right ("climate change is not happening" or " it is not driven by human actions" or "it can be addressed by 'market' solutions."
We may think if we plant a lot of trees, we can ameliorate the problem, but the fact is that we are destroying forests and other wildernesses at an alarming rate, and many pristine areas are now being rendered into industrial parks for land intensive and material intensive "renewable energy," which has not worked to address climate change, is not working to address climate change, and will not work to address climate change. But even if we were not engaged in these massive efforts to destroy forests, it appears, if this paper is correct, that improvements in the mass of biomass on the surface will be offset by decreased soil carbon storage.
From the introduction to the paper:
Plant growth generally increases in response to eCO24,12, with soil nutrients identified as the dominant factor explaining variability across experiments12,13,14,15. The effect of eCO2 on SOC stocks (?soil) is more equivocal. Although the expectation is that SOC will accrue as eCO2 increases plant growth16, a few experiments show increases in ?soil, many show no change, and some even show losses7. The observed variation in ?soil across experiments is puzzling, and there is wide disagreement regarding the dominant mechanisms explaining this variation7,17,18.
A positive relationship between the effects of eCO2 on plant biomass and SOC pools is expected if increased plant production under eCO2 increases carbon inputs (litter) into the soil. Indeed, a positive relationship between inputs and SOC storage is formalized in first-order kinetics16 and is applied in most terrestrial ecosystem models19,20. Because the effect of eCO2 on plant aboveground biomass (?plant) is strongly correlated with the effect of eCO2 on litter production (Extended Data Fig. 1a, r = 0.81) and on root production21, a positive relationship between ?plant and ?soil can thus be expected from first-order kinetics. This hypothesis, however, ignores SOC losses associated with accelerated soil organic matter decomposition sometimes observed under eCO27,18. Plants acquire limiting resources from soils through carbon investment belowground in root growth, exudates and symbiotic bacteria and fungi. Accelerated decomposition of soil organic matter fuelled by plant carbon inputs can enable plant nutrient uptake (the “priming effect”22). The return on this belowground carbon investment is an increase in aboveground biomass production15. However, the priming effect can decrease SOC5. A negative relationship between ?plant and ?soil may thus emerge through the economics of plant resource acquisition.
Here, we evaluate the mechanisms of ?soil, including its relationship with ?plant, by synthesizing 268 observations of ?soil from 108 eCO2 experiments spanning the globe with coupled ?plant??soil data (Supplementary Table 1) using meta-analysis techniques...
Some graphics from the paper:
Fig. 1: Meta-analysis of the effect of eCO2 on percentage SOC across different factors.
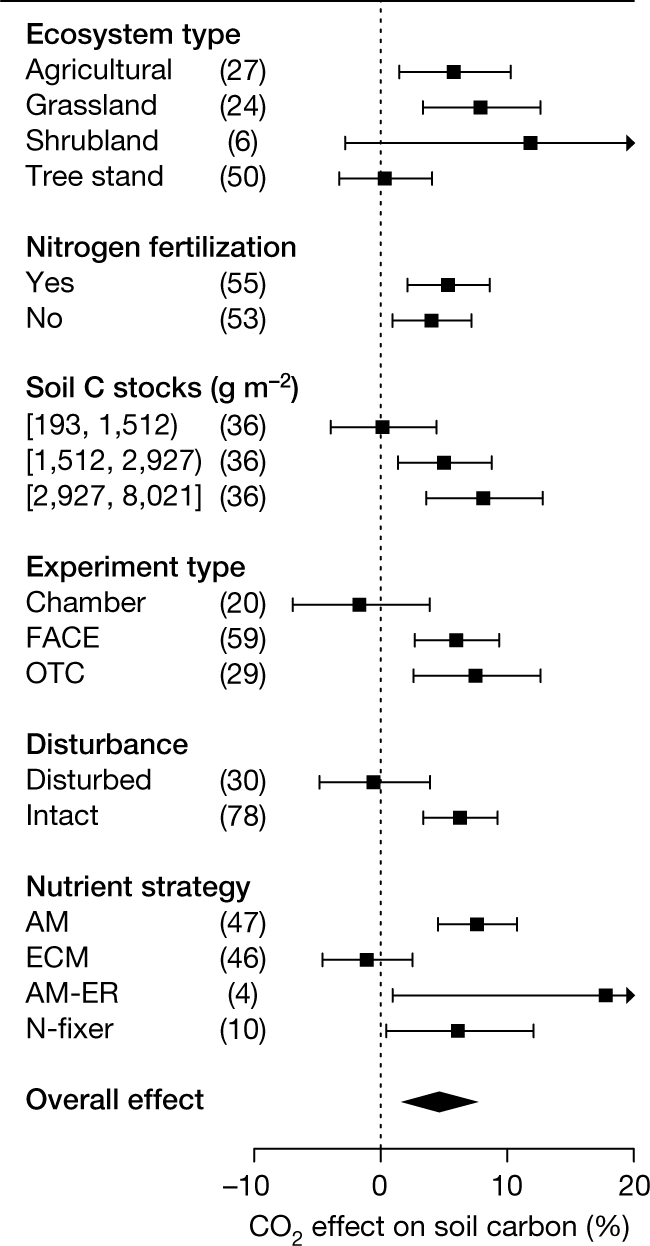
The caption:
The authors state that overall, higher CO2 levels result, according to their meta analysis, in higher soil organic carbon (SOC).
However SOC is negatively correlated with surface biomass:
Fig. 2: Elevated CO2 experiments show an inverse relationship between the effects of eCO2 on plant biomass and SOC stocks due to plant nutrient-acquisition.
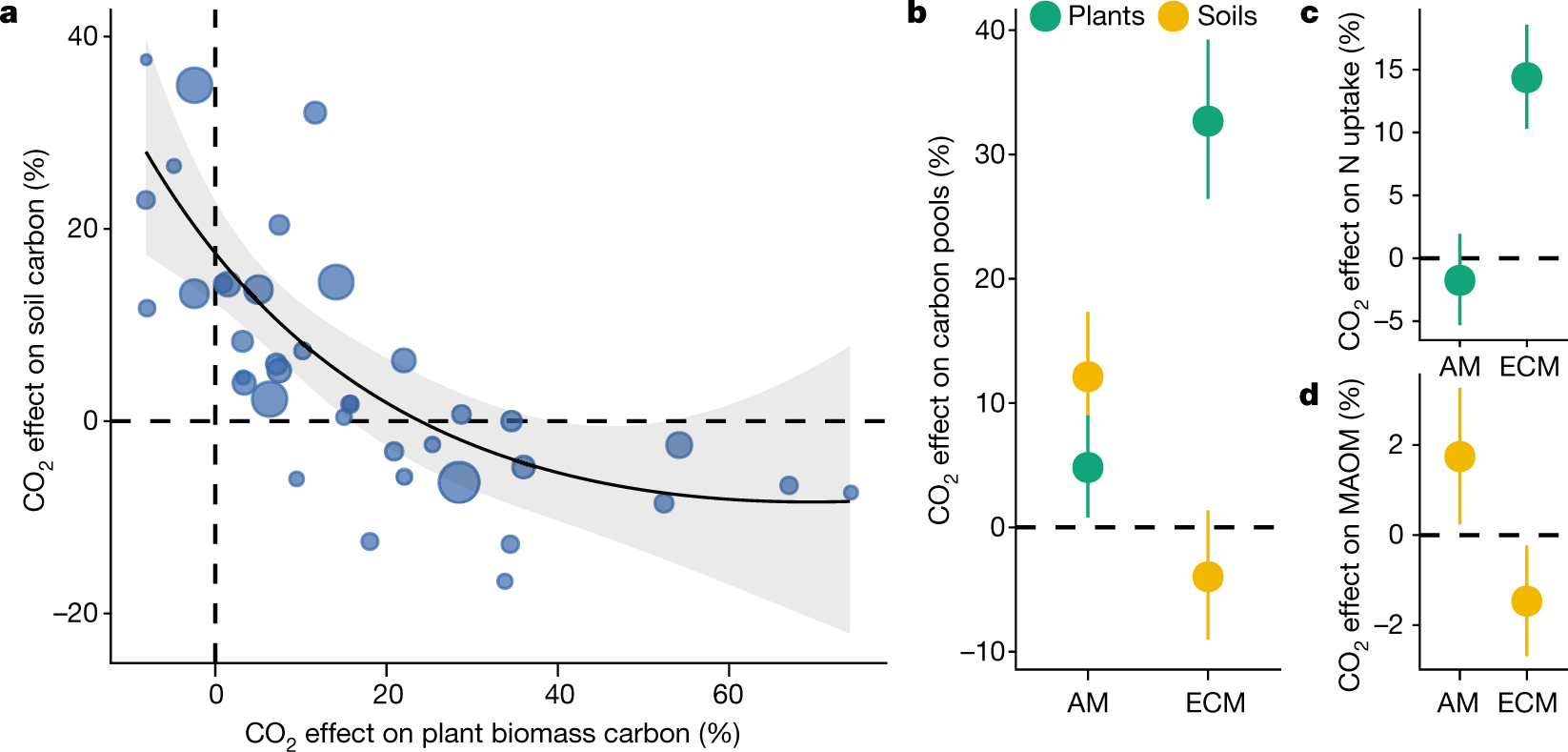
The caption:
Fig. 3: Effect of eCO2 (about 240 ppm) on SOC stocks scaled up from 108 CO2 experiments.
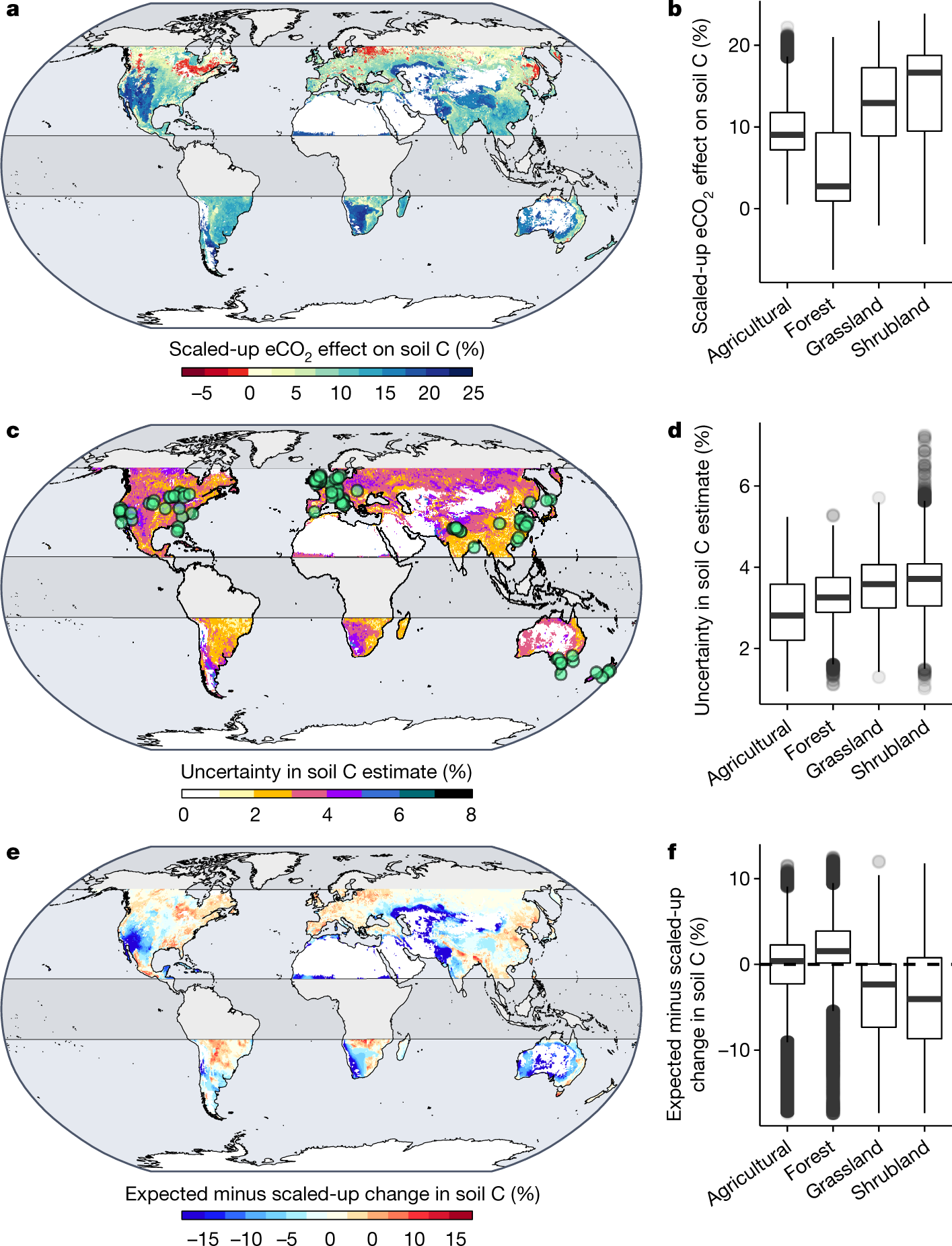
The caption:
Fig. 4: Comparison of modelled and measured relationships between aboveground biomass and SOC responses to CO2.
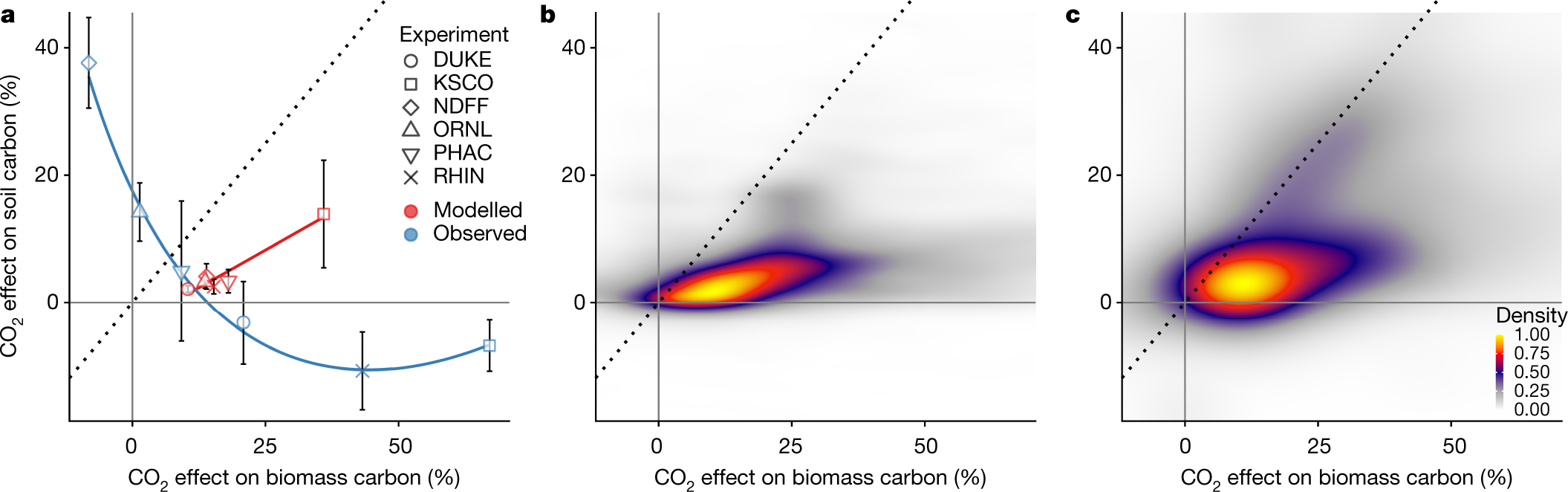
The caption:
Some conclusions from the paper:
This all suggests that forests have a limited capacity to address ever increasing carbon dioxide concentrations. The authors suggest that in terms of soil carbon, grasslands are more efficient than forests.
I note that it took many millions of years for the carbon we've released in the last century to be sequestered by photosynthesis and decay. This should be a sobering thought, given our propensity to believe sunlight alone can save the world. It won't be a sobering thought, but it should be.
Have a nice day tomorrow.
The National Mag Lab: The World's Strongest Magnet.
The video shows the 21 Tesla Magnet, the highest field magnet in the world. (The ion source seems surprisingly generic, but hey, at that field strength, who cares?)
If you go, leave your wallet outside.
The National Ignition Facility.
The world's largest laser is at Lawrence Livermore Laboratory. Cool video.
Dorothy Okello teaches computing to displaced people, and launches programmes to get more women...
...into science, technology and business.
From Nature News:
Engineering a brighter future for refugees and female scientists in Uganda
It's brief, and open sourced. An excerpt:

In this photo, taken in December 2020, I am reviewing students’ progress in a computer networking course at Makerere University in Kampala, Uganda, where I teach electrical engineering and telecommunication policy. The students are learning to configure computers to accommodate Voice over Internet Protocol, or online telephony. We’ve also explored the programming language Python, and technologies for radio and wireless applications...
...As a woman who works in science, I try to promote initiatives that will boost female inclusion in science and technology. One example is the Uganda Women Entrepreneurs Association, which supports aspiring female business leaders.
I’ve also launched projects to get more women into engineering. In 2017, when I was president of the Uganda Institution of Professional Engineers (UIPE), I founded its Committee of Women Engineers, Technologists and Technicians. I also helped to organize training for female engineers that tripled women’s share of the UIPE’s membership to 10% of the total.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,516