NNadir
NNadir's JournalTrends in Nitrate, Arsenic, and Uranium in Groundwater Beneath Irrigated Cropland.
The paper I'll discuss in this post is this one: Using Age Tracers and Decadal Sampling to Discern Trends in Nitrate, Arsenic, and Uranium in Groundwater Beneath Irrigated Cropland (Anthony J. Tesoriero, Karen R. Burow, Lonna M. Frans, Jonathan V. Haynes, Christopher M. Hobza, Bruce D. Lindsey, and John E. Solder, Environmental Science & Technology 2019 53 (24), 14152-14164)
In recent years, I have paid some attention to the destruction of the Ganges River in India - the sacred Ganges among some of the faithful - particularly as it effects the delta nation of Bangladesh. The lower flows have resulted in increased reliance on groundwater for irrigation in Bangladesh, with the concomitant result, since Bangladesh sits on arsenic rich rock formations, with mass poisoning of the population there. One can read about this - among many other places - in another paper in the same journal that features the paper I will discuss: Effectiveness of Different Approaches to Arsenic Mitigation over 18 Years in Araihazar, Bangladesh: Implications for National Policy (Nadia B. Jamil, Huan Feng, Kazi Matin Ahmed, Imtiaz Choudhury, Prabhat Barnwal, and Alexander van Geen, Environmental Science & Technology 2019 53 (10), 5596-5604)
This latter paper contains the following text:
Progress. "Only" 40 million. We need some "renewables will save us" types to come over and make us feel better with percent talk. You know, "Arsenic poisoning in Bangladesh has decreased by 30%!!!!!"
My interest in the Ganges was motivated by that river's role in uranium transport in the Earth's geochemical uranium cycle, when I was doing background research for a post elsewhere on the internet: Is Uranium Exhaustible.
The Ganges transports about 1200 tons of uranium per year to the ocean: Krishnaswami and J. Kirk Cochrane, eds. U-Th Nuclides in Aquatic Systems. Chapter 10, J. Kirk Cochrane and David Kadko, page 293. See also Dunk, R. M., R. A. MiUs, and W. J. Jenkins. Chemical Geology 190, 45-67 (2002)
The transport of naturally occurring elements from the ores in which they are found are obviously affected by environmental chemistry and physical processes. This should be obvious since we use chemistry to isolate elements from their ores. A widely used reagent for the recovery of uranium from its ores - one such ore is used nuclear fuel - is nitric acid, HNO3. Salts of nitric acid, in particular the ammonium salt - an explosive salt that was utilized by the right wing terrorist Timothy McVeigh to blow up a building in Oklahoma City, who may be awarded posthumously the Presidential Medal of Freedom by the anti-Freedom Nazi in the White House (would you really be surprised) - are widely used in agriculture.
It follows that nitrate can mobilize uranium in rocks, and the physical process of pumping water over them, can further exacerbate this extraction process.
From the text of the paper under discussion:
High concentrations of arsenic and uranium occur in groundwater in irrigated areas due to desorption, redox conditions, and evaporative concentration.(4)Irrigation-induced increases in microbial activity and water–rock interactions or the land application of lime often lead to increases in bicarbonate and calcium in groundwater. These increases in bicarbonate and calcium favor the formation of Ca–U(VI)–CO3 complexes that increase the desorption of U from subsurface sediments(5,6) and/or the dissolution of uranium-bearing minerals,(7) leading to higher uranium mobility. The effect of bicarbonate and calcium on uranium mobility is evidenced by the strong correlation between bicarbonate and uranium concentrations in groundwater in a large regional study in Germany(8) and by concordant changes in bicarbonate and uranium concentrations in a national study in the United States.(9)
Infiltrating irrigation water may also alter redox conditions which may affect the mobility and toxicity of contaminants either by affecting the transformation of contaminants to other species (e.g., creating more oxic conditions which may limit denitrification,(10) arsenic reduction(11)), or by causing the precipitation or dissolution of compounds that contain or sorb contaminants (e.g., sorption of arsenic on iron oxides(12)). Irrigating cropland introduces electron acceptors such as dissolved oxygen and nitrate, which can cause the oxidative dissolution of reduced compounds containing constituents of concern (e.g., arsenic, uranium). The correlation of nitrate concentrations in groundwater with selenium and uranium has been suggested as evidence that nitrate may serve as an electron acceptor in the dissolution of selenium and uranium-bearing minerals.(13,14) In one large regional study, the highest concentrations of uranium were observed in manganese and nitrate-reducing conditions,(8) while laboratory studies have found that nitrate is a stronger oxidant of uranium than dissolved oxygen.(15)Arsenic concentrations in groundwater are also often affected by redox conditions due to the release of sorbed arsenic during the reductive dissolution of iron hydroxides.(12,16) While high arsenic concentrations are often associated with reducing conditions, the oxidative dissolution of arsenic-bearing sulfides may also result in elevated arsenic concentration in certain environments.(17,18)
The authors discuss two areas in which the concentration of these two elements and the nitrate ion have been tracked.
The authors chose two relatively arid regions where there is intensive irrigated agriculture:
... Groundwater in the basalt units flows preferentially through the tops and bottoms of individual lava flows, rather than through the less permeable interior.(24)
Figure 1 in the paper is a map of this region.

The caption:
I once encountered a really dumb guy or gal on this website who proudly announced to me that nuclear power is "too dangerous" because a tunnel containing some old chemical reactors at the Hanford Nuclear Weapons plant collapsed. This was in lieu of giving a shit about the 19,000 people who would die each day from the time of his post up to and including today from air pollution. That moron of course, has made it to my wonderful ignore list, but it is notable that the Hanford reservation contains tanks that are rich in nitrate and nitrite and uranium.
You hear these sort of things, but you really don't want to believe that people say these sort of things.
The authors of this paper claim that although the Hanford tanks are well known to contain huge amounts of nitrate and uranium this has had little bearing on their findings:
Reference 27 is here: Water Quality of the Lower Columbia River Basin: Analysis of Current and Historical Water-Quality Data through 1994
Reference 28 is here: USGS Water Data for the Nation
Poking around in reference 28, one can learn of the concentrations of uranium in groundwater in Washington State well upstream from the Hanford reservation. Concentrations of Groundwater Uranium Upstream in the Columbia River Watershed, part of this report: Uranium concentrations in groundwater, northeastern Washington Although the authors of this report and the authors of the paper under discussion all work for the USGS, the data does seem to suggest that uranium concentrations in ground water in Washington State are not a function of the existence of the Hanford tanks, although credulous anti-nukes will not believe it.
In any case, the authors second study area is in Nebraska, also in an irrigated region:
This study area has a humid, continental climate,(20) receiving an average of 68 cm of precipitation each year.(31) Cropland, primarily corn and soybeans, is prevalent in the study area, with most of this cropland receiving irrigation. Rates of recharge from precipitation are estimated to be 14.2 cm/yr, with irrigation recharge estimated to be 6.4 cm/yr.(31) While groundwater has been used for irrigation in the High Plains area since the late 1800s, intensive irrigation did not occur until the mid-1900s. Irrigated cropland began to increase dramatically in the 1950s in the Nebraska portion of the High Plains aquifer; (32) climate change may result in future increases in irrigation demand in this area.(33)
Fertilizer applications have increased markedly since 1950, resulting in dramatic increases in nitrate concentrations in recharging groundwater in recent decades.(3,34) Stanton et al. (2006) sampled shallow groundwater from 30 wells in the High Plains aquifer in 2004 and determined that nitrate concentrations in shallow groundwater ranged from 2.0 to 106 mg/L as N, with a median concentration of 10.6 mg/L.(29) Nitrate-N isotope ratios in agricultural recharge suggest that fertilizer is the primary source of nitrate in groundwater recharge in this area.(3)
The study area:

The caption:
Figure 2. Site map of High Plains aquifer wells sampled for this study. Map is from Arnold et al. (2018, https://pubs.er.usgs.gov/publication/ds1087). Identification numbers correspond to hpgwvfps1 listings in Table 1 of Arnold et al. (2018), where water quality and construction details are provided for each well.
The age of the water tested is determined by the concentrations of the radioactive hydrogen isotope tritium injected into the planetary atmosphere by nuclear weapons testing, particularly by hydrogen bombs:
Groundwater samples were analyzed for tritium at all sites and sulfur hexafluoride (SF6) at the High Plains sites. Samples were characterized as premodern, mixed, or modern using the tritium category method.(46) A brief discussion of the tritium category method is provided below, with details provided elsewhere.(46) The tritium category method relies on variations in concentrations of 3H in groundwater given the temporal and spatial variation of 3H in precipitation.(47)3H concentrations in precipitation were at low, naturally occurring levels before 1953 but subsequently increased rapidly due to above-ground nuclear bomb testing (Figure 2b in Lindsey et al., 2019).(46)3H concentrations in precipitation remained high for many years, only returning to near prebomb concentrations in the past decade. However, 3H concentrations in groundwater that recharged prior to 1953 are much lower than recharged in the postpeak era due to the decay of 3H between 1953 and the sample collection date. This decayed concentration of recharge just prior to 1953 represents the upper threshold concentration of what is classified as premodern water. Conversely, a 3H concentration in groundwater that is greater than the lowest decayed concentration expected from precipitation during the postpeak period must have recharged after 1952 and is classified as modern water. Lastly, 3H concentrations that are between the upper and lower threshold are the result of a mixture of modern and premodern water and are termed mixed water. Groundwater ages of modern samples at the High Plains site were further refined by measuring SF6 concentrations in groundwater samples and relating these concentrations to atmospheric inputs.(48)
SF6 is a persistent greenhouse gas, with a global warming potential of 23,900 relative to CO2. It is totally anthropogenic, and has been industrially synthesized to replace PCB's in electrical transformers and to make those wonderful insulated solar windows in modern McMansions. Thus its presence in water is a time marker, given that the gas has only existed in prominent concentrations in recent times; it does not occur naturally.
Here are tables of results from the paper:

Some other graphics:

The caption:

The caption:

The caption:

The caption:
Figure 6. Boxplots of nitrate concentrations in modern groundwater as a function of irrigation water source at the Columbia Plateau site. Samples collected in 2014. Number above each plot indicates number of samples used in calculation. Some outliers are not shown.

The caption:

The caption:
According to the authors, it does appear that while the concentrations of these analytes has increased since the beginning of irrigation, but the concentrations have stabilized since the early 2000's.
There is some discussion in the paper of the role of phosphate, which along with nitrate, is an element of commercial fertilizers on which the world food supply depends:
Although phosphate here is discussed in connection with its cogener anion arsenate, it is well known that uranium is frequently a constituent of phosphate minerals and historically, before minerals were discovered having a higher concentration, phosphate mines were often considered as uranium ores in addition to phosphate ores. As a result of not removing uranium from phosphates, uranium is widely distributed on agricultural fields as a constituent of fertilizers.
A recent publication, albeit from a few years back, has examined the question of whether uranium should be mined from phosphate: To Extract, or not to Extract Uranium from Phosphate Rock, that is the Question (Haneklaus et al., Environ. Sci. Technol. 2017, 51, 2, 753-754)
It's open sourced; anyone can read it. It contains this text, which is not hostile to the only technology with even a remote chance of addressing climate change, nuclear energy:
"ISL" is a technology known as "in situ leaching."
I have argued in many places, here and elsewhere, that uranium mining need not be necessary at all for centuries. If we simply convert the uranium already isolated into plutonium, all of the world's energy needs for all purposes, can be met using uranium (and waste thorium from lanthanide mine tailings) already mined. However it is also possible to recover uranium in remediation schemes for groundwater with mobilized uranium as a result of nitrate leaching, such as that resulting from irrigation or - in the case of abandoned "fracking" fields like those in California and Pennsylvania, from abandoned oil and gas wells. (Coal ash is also a potential source of uranium.) Recovery of this type of uranium as a side product of remediation of natural uranium mobilization as a result of agricultural or industrial processes may well extend the time that the use of uranium can prevent the need for any energy mining, including the disastrous mining of oil, gas and coal, and for that matter, steel, copper, lanthanides and bauxite to support the pixilated so called "renewable energy" industry.
Nuclear technology has the capability of offering sustained high temperatures, and thus has the possibility of running at much higher thermodynamic efficiency than any other technology, meaning that even at current levels of energy production, running about 600 exajoules per year, the benefits of energy might be extended more broadly to those who lack it. (On the other hand, Jevon's paradox does offer a warning counter intuitive caveat on the benefits of efficiency.)
To return to the paper cited at the outset, here is the authors' concluding summary of the paper under discussion:
These findings suggest several areas that should be prioritized when monitoring groundwater beneath irrigated cropland. First, high nitrate concentrations in shallow modern groundwater have been sustained for decades and pose a future risk to deeper groundwater used for drinking water. Given the oxic conditions of these aquifers, nitrate concentrations may be expected to increase in older modern water as nitrate in the shallow portion of the system continues to migrate into deeper portions of these aquifers. Second, increased monitoring for trace metals in shallow groundwater beneath irrigated areas is warranted, as these contaminants may be mobilized by changes in water chemistry: increases in bicarbonate may mobilize uranium and increases in phosphorus may mobilize arsenic. Third, areas that use groundwater for irrigation may have an elevated risk of high nitrate concentrations due to the repeated dissolution of land applied fertilizers during recirculation.
I trust you are enjoying your weekend.
I'm very concerned that my cat will vote for Trump.
She has a brain the size of a walnut.
Material balance evaluation of pyroprocessing for minor actinide transmutation nitride fuel.
The paper I will discuss very briefly is this one: Material balance evaluation of pyroprocessing for minor actinide transmutation nitride fuel. (Sato et al., Journal of Nuclear Science and Technology. 2020, VOL. 57, NO. 3, 224–235.)
The actinide nitrides are very attractive nuclear fuels because of their high thermal conductivity and the ease by which they are reprocessed. During his quest to learn how to fix nitrogen, Fritz Haber explored a uranium catalyst for the purpose. Uranium nitride when exposed to water rapidly decomposes to give aqueous ammonia and uranium oxides. (Haber was able ultimately to utilize hydrogen and ammonia to accomplish the task, which was then industrialized by the German chemical engineer Carl Bosch. The invention allowed Germany to sustain itself for four years in the first world war without access to Chilean salt peter.)
The property makes it very convenient to reprocess nuclear fuels.
The paper above is about an ADS system, an accelerator driven system, which uses neutron spallation by a beam of high energy protons to fission actinides in a subcritical state. Although my son worked this summer at a neutron spallation facility, I'm not a fan of ADS reactors for various reasons, but what is interesting about this paper is the pyroprocessing of used nuclear fuels for separation of valuable elements therein.
From the paper's introduction:
Nitrogen-14, 14N, when bombarded with high energy neutrons, undergoes a nuclear reaction in which a proton is ejected from the nucleus, giving carbon-14, 14C. In general, most writers think that this is a bad thing, since carbon-14 is radioactive. However, I disagree. Carbon 14 has a very low neutron capture cross section compared to the two other carbon isotopes, the non-radioactive carbon-12 and the other non-radioactive (but rarer) isotope carbon-13. 14-C is a slightly less efficient moderator than natural carbon, meaning that regions of fast neutrons and epithermal neutrons are easier to maintain in breeder blankets where actinide 14-C carbides are used. I think we would be better off with more 14C, not less, but that's just my opinion.
As I've noted in this space, all of the transuranium isotopes have a critical mass in a fast neutron spectrum, and therefore it is not actually necessary to treat them with neutron spallation, as in an ADS system, but no matter. The processing is what is interesting, not the mechanism of fission.
Here's the flow chart for the process described:

There are several references to "waste" here. In my opinion, they are all unnecessary. Nothing that is useful is waste, and given the nature of our intractable chemical pollution problem, radiation can serve to remediate some very severe cases; in fact in some cases, it may be the only tool for remediation. I'd stay away from that zeolite thing, myself. Under these circumstances, this particular molten salt has a number of things that don't recommend it, one being the presence of chloride and the other being the problem of tritium being generated from lithium. (On the other hand, if people ever get fusion reactors to work, tritium will be a valuable fuel.)
To my way of thinking, this is not an ideal pyroprocessing approach, but it's nice one at which to look to stimulate thought.
A table of fuel composition on loading and on discharge:

Recovery of metals in the liquid cadmium cathode:

Decay heat:

The isotopic mix of the plutonium is actually quite wonderful. This is an example of proliferation-proof plutonium, because of it's heat load.
Nice stuff.
Esoteric, but this sort of thing represents the only path out of climate change in my opinion.
There are many, many, many other possible similar processing schemes. This is just one.
Enjoy your Friday.
John Quincy Adams. The first one I remember was Polk vs. Clay, and my first vote was for...
...Winfield Scott.
I was really pissed off when that asshole Franklin Pierce won the election, which lead to Buchanan...
Of course, Buchanan was, until Trump, considered the worst President ever, so it kind of gives one hope, since Lincoln came along and cleaned up the mess and actually made the country better than it was when those two asses before him held the office. We'll need another Lincoln, but we could do without Civil War II.
I note that the United States had a President before Trump who was also a traitor, albeit not to a foreign power as Trump is to Russia.
Ex-President John Tyler served in the Confederate government, and thus was a traitor to his country.
Premature mortality related to United States cross-state air pollution.
The paper I'll discuss in this post is this one: Premature mortality related to United States cross-state air pollution (Barrett et al., Nature 578, pages 261–265 (2020))
Ultimately the most dangerous fossil fuel wastes will be those associated with climate change, but dangerous fossil fuel waste has been killing people all over the world for a century. The de-industrialization of the United States, along with political laws associated with automotive and power plant emissions, the latter being part of the Clean Air Act now in the process of being gutted, and the substitution of dangerous natural gas for dangerous coal in the United States has lead overall to a decrease in the deaths here; we now export air pollution deaths to import trinkets for our bourgeois lifestyle, but people still die here from air pollution.
This paper is an attack on the ever popular theory advanced by the racists who dominated the Democratic Party in the 19th century, and in the big switch, the Republican party in the late 20th and early 21st century. It shows that it rains particles in the lungs in the just and unjust alike.
From the abstract, which is surely open sourced:
From the introductory text in the body of the paper:
Combustion emissions constitute the largest source of anthropogenic emissions in the USA, and therefore contribute to the formation of PM2.5 and ozone2. The health impacts attributable to these emissions have been estimated in various studies6,13,14, with estimates varying between 90,000 and 360,000 early deaths per year. In the context of the Environmental Protection Agency (EPA) Cross-State Air Pollution Rule (CSAPR) and individual state regulation, measures to further reduce the health impacts of pollution would benefit from a greater understanding of which sectors and which states are responsible for the health impacts in every other state.
The paper's goal:
Some graphics from the paper:
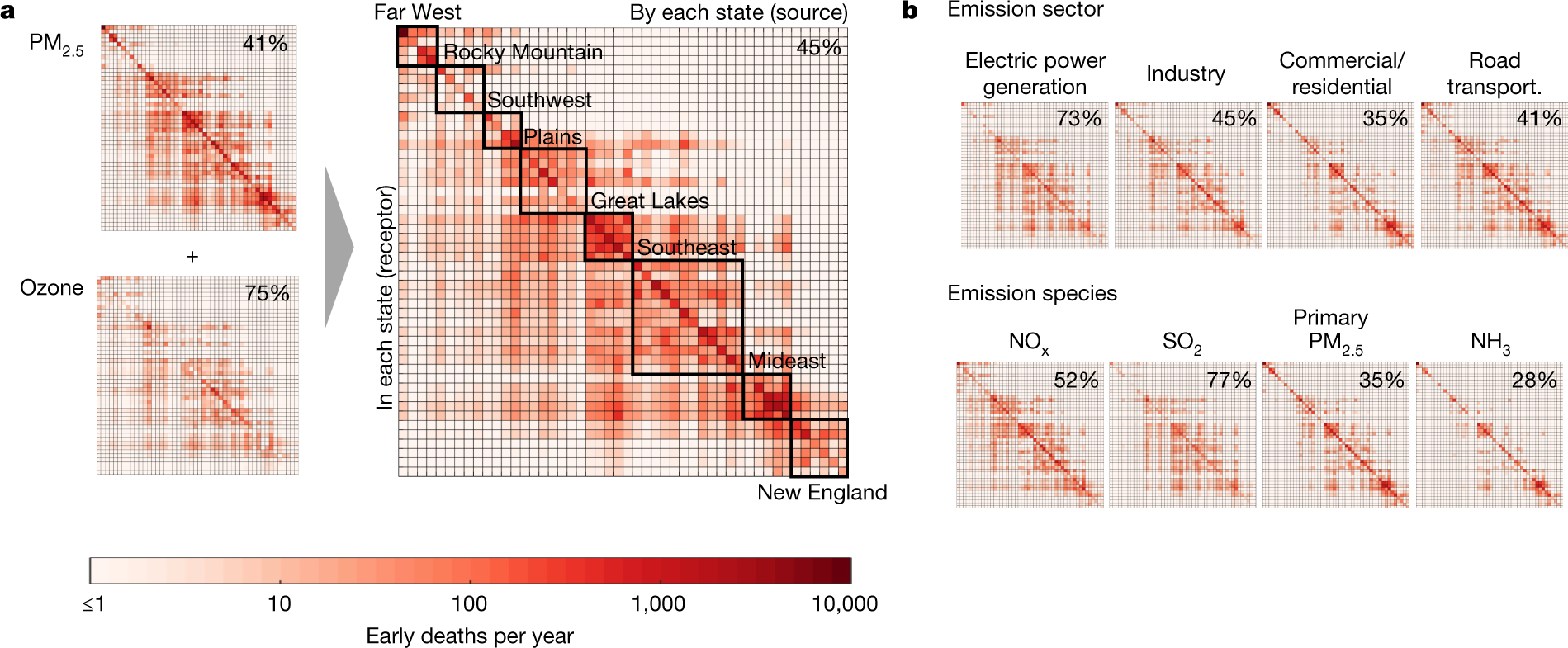
The caption:
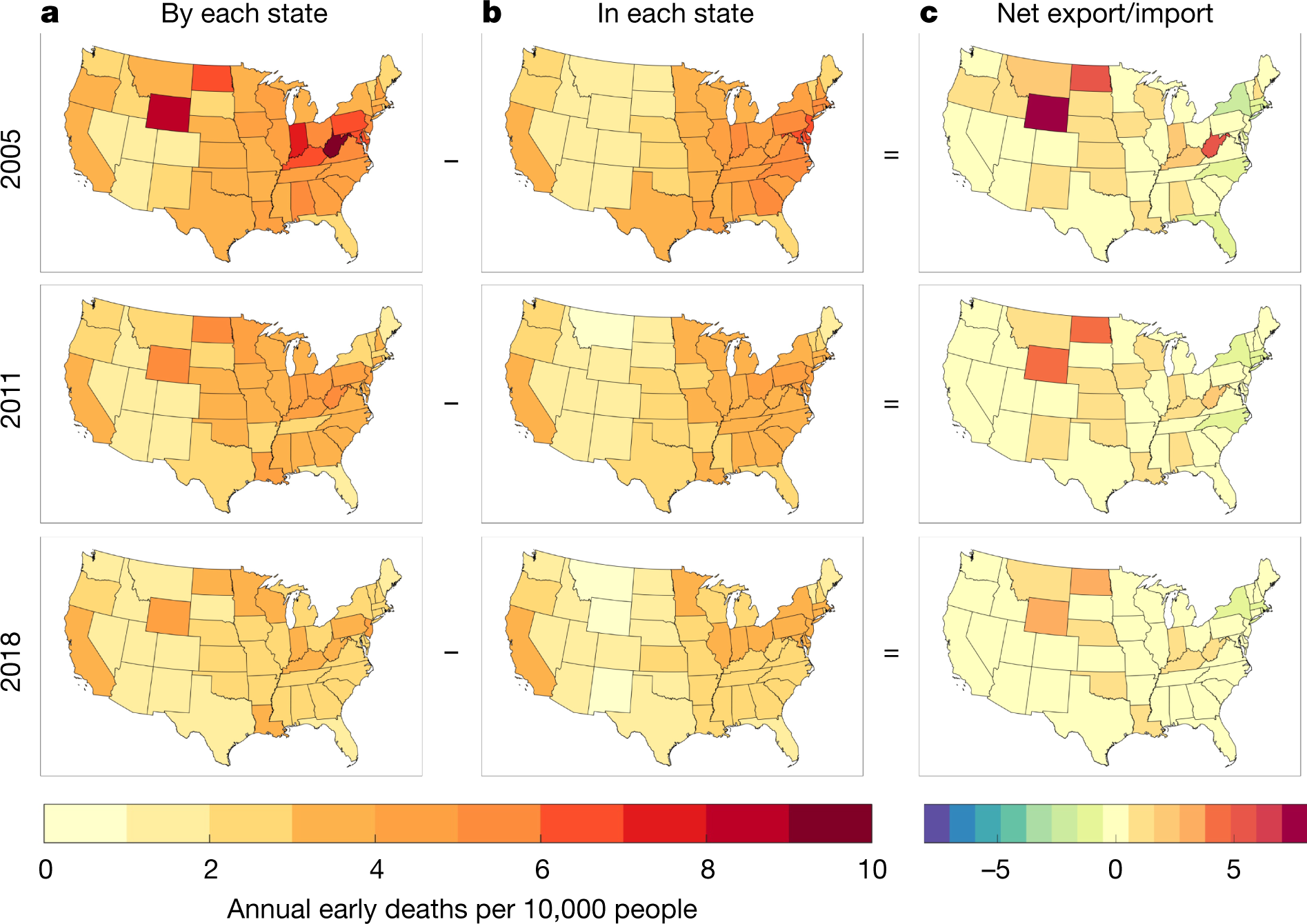
The caption:
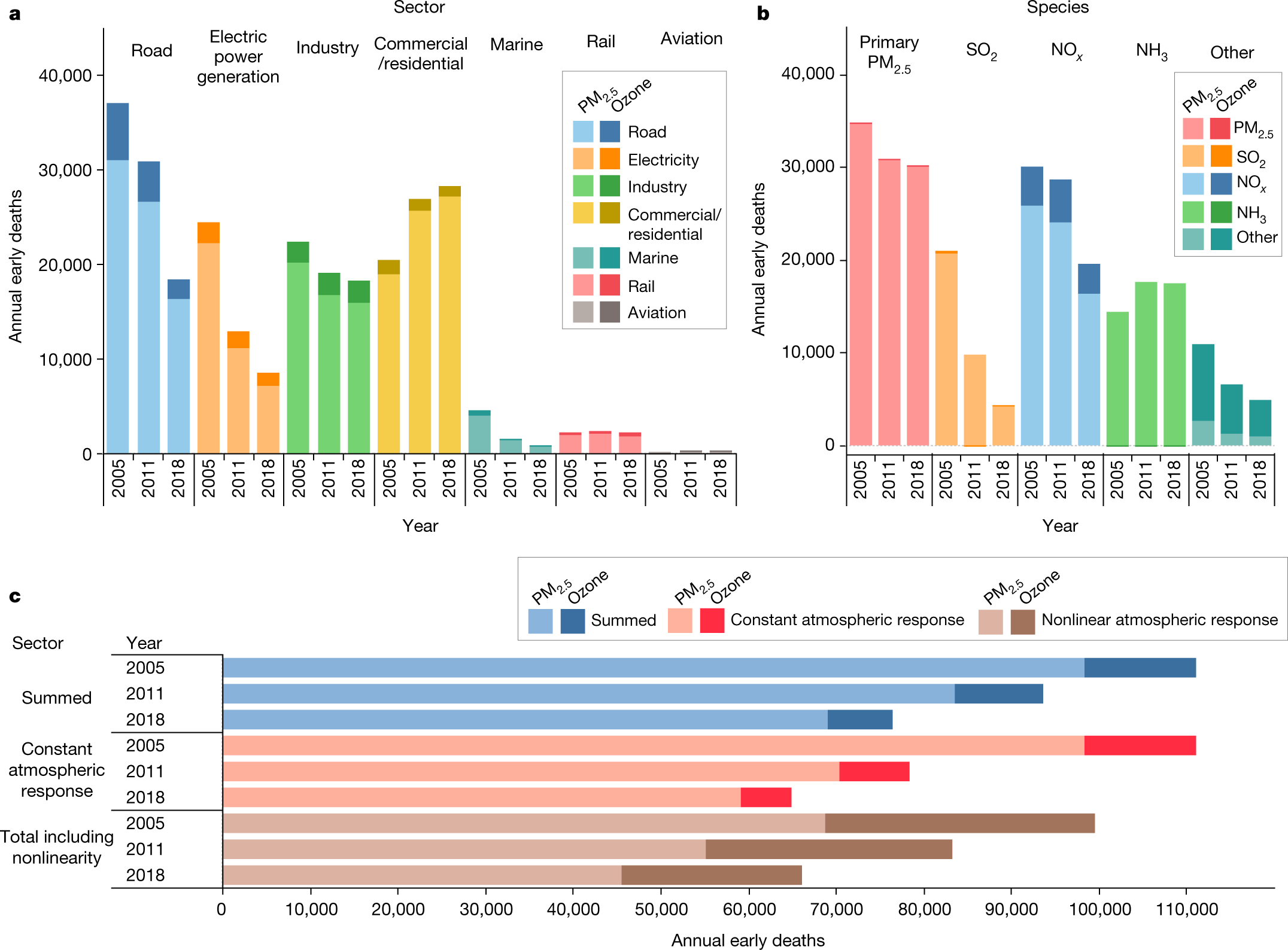
The caption:
Some more text:
Ammonia-attributable impacts increased by around 21% between 2005 and 2018. This difference was driven by an increase in the sensitivity of PM2.5 exposure with respect to a unit of ammonia emissions between 2005 and 2011. Owing to the decline in the importance of SO2, ammonia impacts went from being the fourth-greatest to the third-greatest contributor to total impacts over this period, increasingly close to the contribution of NOx species. NOx remained the second-greatest contributor to impacts from 2005 to 2018. Despite the roughly 50% reduction in total NOx emissions between 2005 and 2018, impacts attributable to NOx reduced by only around 35% between the two years. This is largely due to the increased sensitivity of PM2.5 formation to NOx emissions between 2005 and 2011, as noted previously23
Here in the United States we think that coal is dead because we've begun burning gas by utilizing a system by which we pulverize the bedrock of the continent irreversibly and in the process, probably destroy or severely damage the groundwater supply for all generations that come after us. Don't worry, be happy. We're green.
It is true that dangerous natural gas can cause particulates via Boudouard Chemistry, but overall the particulates are lower for dangerous natural gas than for dangerous coal. However the fastest growing source of energy on the planet as a whole - not that people in our racist country care about people in other countries where poor people make and/or recycle our precious stuff - has been coal.
We couldn't care less.
Don't worry; be happy. The steel for all those wind turbines we've bet the future of the planet on will be made in Chinese blast furnaces, and not in Pennsylvania. Here in New Jersey, we no longer have to breathe that shit from steel plants. They're closed and they're rotting. Pennsylvania just gives us our "clean" "transitional" natural gas now.
I trust you had a pleasant weekend. Mine was pretty good; not all the science I read was this depressing.
Family Redux: I had a wonderful kind of angry bitter political argument with my son.
Once when we were first married, my father and my stepmother - who had been married to my father for less than six years - came to visit us in California.
My father and I hadn't seen each other for about a year or so at the time, and we loved each other very much.
We had this big deal screaming argument with each other over the war in Vietnam - which had been over for more than a decade at the time - and my stepmother told my father that she thought they were going to get thrown out of our apartment and would have to go to a hotel.
My father just laughed and remarked about how proud he was of how I kept pulling books off my bookshelves to bolster my hotheaded argument.
The next morning we drove down to San Diego harbor so he could reminiscence about his last visit to San Diego, where he spent a day loading ammunition on his aircraft carrier during World War II.
It was a wonderful visit. I very much miss the ability to have heated political arguments with my father, who died in 1993.
The other night I had the chance to have a heated argument with my son over Michael Bloomberg. It was like the old days.
My wife - who'd been there for that San Diego argument - remarked on how heated the argument was.
"It's a family tradition," I said.
My son, who loves stories about his grandfather - who he never met - and I just laughed.
Life is beautiful, and then you die.
The art of misleading the public
This book review is in the current issue of the scientific journal Science.
The review is by Sheril Kirshenbaum.
The book is:
The Triumph of Doubt: Dark Money and the Science of Deception David Michaels Oxford University Press, 2020. 344 pp.
The Art of Misleading the Public Science 14 Feb 2020: Vol. 367, Issue 6479, pp. 747
A large excerpt of the review:
Michaels is the quintessential voice on the influence of special interests in policy-making and government inaction. An epidemiologist and professor of environmental and occupational health at George Washington University, he spent 7 years leading the U.S. Occupational Safety and Health Administration (OSHA) under President Obama and previously served as President Clinton's assistant secretary of energy for environment, safety, and health.
His book offers account after account of unethical bad actors working against the public good on issues ranging from asbestos to climate change. Powerful firms and individuals seeking personal gain repeat the tactics of a well-worn playbook of denial and misdirection proven effective by Big Tobacco more than 50 years ago. Michaels pulls no punches, naming the corporations and people responsible for fraud, deception, and even what he terms “climate terrorism.” He reveals the dirty ways that industries have succeeded at shaping their own narratives regarding safety and health by producing articles and diversions designed to deny and distort science while confusing the public.
When a Boston University brain study found that 110 of 111 National Football League (NFL) players' brains showed pathologies consistent with the rare disease chronic traumatic encephalopathy (CTE), the NFL hired its own conflicted scientists to counter and discredit these troubling findings. When reports from the International Agency for Research on Cancer, the U.S. National Toxicology Program, and the World Health Organization independently linked alcohol consumption to certain cancers, the alcoholic beverage industry claimed that these associations were not real and doubled down on its messaging that moderate drinking is good for us. When the opioid epidemic hit the United States, ravaging families and communities, well-documented evidence suggests that drug companies suppressed research and misrepresented the clear science demonstrating that opioids are addictive and easily abused.
What is most striking in The Triumph of Doubt is that Michaels is not merely reporting on how corporations and industries manufacture uncertainty. Rather, he provides an insider's perspective on the machinations taking place in the nation's capital, in courtrooms, and across the country...
Physico-chemical properties of Chernobyl "Elephant's Foot" Lava.
I'm on a couple of technical news feeds at my job and an article in one of them caught my eye since I am interested in all things involving nuclear energy. The news item is here: Innovative Material Could Help Clean Up Chernobyl and Fukushima.
Whenever one reads a press release about science with the word could in the title, one's "critical thinking required" alarm should light up and start buzzing loudly. I would argue that 95% of the time, or perhaps more, when this happens one should expect to read a distortion, wild inflation, or overly optimistic or overly pessimistic interpretation of a laboratory finding that has little to do with what actually happened in the laboratory.
I am an old man. For my entire adult life, I have been reading how so called "renewable energy" could power the entire world, "by 1995," "by 2000," "by 2010," "by 2015," "by 2020..." and so on.
Reality:
Here is a table of sources of energy taken from the data found in the International Energy Agency’s 2017, 2018, and 2019 Editions of the World Energy Outlook published annually:

A table of changes:

Sources:
2019 Edition of the World Energy Outlook Table 1.1 Page 38] (I have converted MTOE in the original table to the SI unit exajoules in this text.)
IEA 2017 World Energy Outlook, Table 2.2 page 79
Sometimes, I'd guess maybe 10% to 15% of the time, one is inspired to actually look at the original source article - if, in fact, one has been published - to find out what is actually being stated by the scientists who did the work, without added journalistic or marketing spin.
In the case of the above cited news article, here is the actual paper to which the news item actually refers:
Synthesis, characterization and corrosion behaviour of simulant Chernobyl nuclear meltdown materials. (Hyatt, et al, npj Materials Degradation volume 4, Article number: 3 (2020))
It is open sourced; anyone can read it.
It does contain the following statement in the abstract:
"LFCM" is defined above in the abstract as "Lava-like fuel containing materials."
The event at Chernobyl was, and always will be, the worst case for nuclear reactor technology. All the money spent to "clean up" Chernobyl will save very few lives, because 34 years later, very few lives are now at serious risk.
Recently elsewhere in this space I wrote this in response to a comment:
Let's say that the death toll of air pollution averaged, over the last 34 years, five million people per year, a lower rate than what is currently understood.
That works out to 170,000,000 deaths from air pollution in the 34 years since Chernobyl.
Of course, the fact that we don't pay attention to one, and microexamine the other makes no difference in the actual numbers.
And then there's climate change. Do you grasp how serious, how much death and destruction will be involved in comparison to Chernobyl?
Here are some things that have killed more people than 60 years of nuclear operations: Automobiles, aircraft, fatty foods, water, house fires...
Do we routinely assume that cars, aircraft, fatty foods, water and houses are "too dangerous?" Do we say any of these things should be phased out? (For the record, I do believe that cars should be phased out, but that's just me.)
I'm a scientist. I am trained to think critically. In general this means rejecting journalistic impressions, which are often geared at making people not think critically but rather in emotive and/or sensationalist terms.
Look at politics. "But her emails..."
I look at journalism about nuclear energy in exactly the same way, "...but her emails..."
We could save more lives that we propose to spend to "clean up Chernobyl and Fukushima" to some absurd standard of safety if we spent the same amount of money to stop destroying the planetary atmosphere. That's my could statement.
Nuclear energy, overall, saves lives by preventing dangerous fossil fuel waste, including but limited to air pollution, from killing people, which it does continuously, with and without accidents occurring, that is, during normal operations.
Reality.
From the above data from the IEA, despite all the hoopla about solar and wind energy saving the world, all the cheering, all the "could power 100% of world energy by 'year such and such'", after an "investment" of trillions of dollars devoted to wind and solar every decade, coal has been the fastest growing source of energy on this planet in the 21st century.
Again, it's open sourced; you can read all about the "LFCM simulant" in the original paper, if you're interested. I found looking through the references in the paper to be interesting, since the references refer to some of the real "Chernobyl Lava" that has been recovered from the reactor.
The real Chernobyl lava is interesting though. I could not access some of the papers through Google Scholar, but I was able to download reference 17, which is not, I believe, open sourced. Here it is:
Physico-chemical properties of Chernobyl lava and their destruction products (Andrey A. Shiryaev, et al. Progress in Nuclear Energy 92 (2016) 1040-118)
It contains this text:
Here is a picture of the "elephant's foot:"

The person in this picture was of course, in great danger; it may be "Mr. Vladimir Zirlin of the V.G. Khlopin Radium Institute" referenced in the text above. The grainy nature of the picture is almost certainly connected to radiation exposure of the film during the trek to take the photograph. If this is Mr. Zirlin, he was still publishing papers as late as 2012.
Development of New Generation of Durable Radio-luminescence Emitters based on Actinide-doped Crystals (Zirlin et al., Procedia Chemistry Volume 7, 2012, Pages 654-659).
He seems to have lived at least 22 years after taking the picture, and, in fact, was still working 22 years after taking the picture. It would hardly be surprising however, if his life has been significantly shortened by the act of taking the picture than if he had not taken the picture, and bravely carried the samples out of the reactor for analysis, whereupon they were sealed in an acrylic resin.
Anyway, let me return to the interesting Progress in Nuclear Energy paper. It begins with a description similar to those one can read in many places both in the general and the scientific literature, with more or less detail. These descriptions are very popular and wide spread in both the professional and general news, as people are far more interested what's going on in Chernobyl since 1986 than they are in the 170,000,000 deaths from air pollution since 1986 that I postulated above.
Here is the introduction:
In the RBMK reactors the reactor basement plate is a cylinder 14.5 m in diameter and 2 m in height, filled with serpentinite with bottom and top steel lids interconnected by stiffening ribs and water tubes. During the explosion a 100-110_ sector of the basement plate was pushed approx. 4 m down, merging the reactor shaft with a former sub-reactor room 305/2 (e.g., Arutyunyan et al., 2010). The amount of nuclear fuel in the room 305/2 is estimated at 65-80 tons of UO2 (Borovoi et al.,1998). Before and shortly after the explosion the fuel reacted with zircaloy and later with construction materials (sand, concrete, serpentinite, steel), leading to the formation of so-called lava-like fuel-containing materials (LFCM) or Chernobyl “lava” (Burakov et al., 1994, 1997a,b; Ushakov et al., 1997). Several days after the accident considerable fraction of the initial lava pool spread into other rooms of the reactor building (Burakov et al., 1997a), forming vertical and horizontal flows which solidified into a highly radioactive glassy material with inclusions of high-uranium zircon crystals (Zr1-xUx)SiO4, particles of molten stainless steel, uranium oxide dendrites and grains, and particles of Zr-U-O phases (solid solutions in the system of UO2-ZrO2). Several varieties of the lava are known (e.g., Anderson et al., 1993; Borovoi et al., 1990, 1991a, 1991b; Burakov et al., 1994, 1997a,b; Pazukhin, 1994; Pazukhin et al., 2006; Savonenkov et al., 1991; Trotabas et al., 1993): 1) brown lava; 2) black lava, and 3) much less abundant and less studied polychromatic lava. On the lower levels of the reactor building the flow of brown lava entered water in the bubbler tank forming pumice-like material (Borovoi et al., 1991a; Trotabas et al., 1993). Controversy still exists about the total amount of uranium in all “lava” streams in comparison with initial fuel inventory. Estimates vary from 9-13% (Kiselev and Checherov, 2001) to >80% (Arutyunyan et al., 2010) of total amount of the ChNPP fuel; the rest is believed to remain in inaccessible premises of the reactor, possibly as fuel rods fragments.
Since 1986, and 1990, when the lava samples were first collected at Chernobyl, there have been huge advances in analytical chemistry, and the purpose of the paper is to utilize these lava samples using this new technology:
In addition, some new samples were acquired:
Aerosol particles were collected in 2010-2014 at the distance of 20-30 cm from the lava heap in room 012/7 (level 0.0 m, the first floor of the Bubbler tank (Borovoi et al., 1991a)) using a pack of three Petryanov filters with different particulate retention sizes mounted on the nose of the air blower Н810 RadeCo operating for 2 h at a pump rate 100 dm3/min. Daily variations of the air temperature in this room are negligible, annual variations are within 4С -9С in winter and 13-С in summer). Chemical and radionuclide (e.g., 137Cs/241Am) composition of the particles collected is consistent with composition of the heap (Pazukhin et al., 2003; Ogorodnikov et al., 2013).
2.3. Spontaneously detached individual sub-millimeter particles
These chips were collected in 2013-2014 on the planar cuvette placed for 6 months on the floor 0.50 m in front of a lava heap in room 012/7 (see chapter 2.2). These particles are of particular interest, since their detachment from the lava accumulation appears to be spontaneous. The particular lava agglomeration is mechanically heterogeneous: the internal part is highly porous (pumice-like or granulated, see Fig. 1G, H) since it was formed when hot brown lava stream entered in contact with water in the Bubbler tank, whereas the outer shell is glassy due to rapid quenching (Borovoi et al., 1991a; Pazukhin et al., 2003). The glassy shell was partly broken by researchers. The exact origin of the studied particles e the heaps’ shell or interior or even destruction of eventual pieces of pumice observed in this room is unclear.
There is reference in the excerpts above to several isotopes of interest in connection with the Chernobyl event, specifically, 137Cs and 90Sr. These two elements, cesium and strontium, and in fact their salts and/or oxides, are highly volatile at the temperatures described during the Chernobyl meltdown, and it is well known that they were widely distributed across Europe in detectable amounts. (It should also be noted that being radioactive, they can be detected as extremely low levels, not all of which represent a severe health risk.)
I have long been following with interest the behavior of environmental loads of Cs-137 in particular, since its volatility suggests some interesting possibilities in nuclear reactor engineering that may be of use when future generations if and when they find the where-with-all and resources to clean up the dangerous fossil fuel disaster that we have left for them, in deep contempt for their lives, something that will only be possible using nuclear technology, given the high energy to mass ratio of nuclear fuels.
For example, before being banned from Daily Kos for telling the truth, the truth being that opposing nuclear energy is akin to murder, I wrote this somewhat sardonic piece in 2010: Post-Chernobyl Radionuclide Distributions in an Austrian Cow. At that time, in 2010, 45.6% of all the Cs-137 released by the Chernobyl accident had decayed to non-radioactive Ba-137. As of this writing, about 54.3% has decayed, 45.7% remains.
Neither Austria nor the rest of Europe has been depopulated by eating cows containing Cs-137 since 1986. (Cows all around the contained Cs-137 well before 1986 from open air nuclear weapons testing. In fact, about 17.7% of the Cs-137 released by the 1945 Trinity nuclear weapons test in 1945 is still radioactive. It seems to be inevitable that New Mexican cows have detectable Cs-137 in them.) Eating cows, by the way, is generally bad for you. More people have died from fatty foods since 1986 than have died from Chernobyl, way more people, hundreds of millions of people in fact. Chernobyl or not, no one ever proposes banning cows because eating them is "too dangerous."
Anyway. Anyway. Anyway.
Here are some tables on the elemental composition of the Chernobyl lava:


A few elements listed here have long lived radioactive isotopes that may be detectable in the samples: They are zirconium (Zr-93 as a fission product and from radioactive induction in Zr-92 in structural materials), iron (Fe-60 from radioactive induction of short lived Fe-59, in turn from the induction of Fe-58) and, of course, uranium, which has been radioactive since the formation of the Earth, and which would have been radioactive whether or not it had ever been in the core of the Chernobyl Unit 4 reactor.
The specific activity - the number of radioactive decays per gram - of none of these elements is particularly high; in every case they are way lower than the specific activity of cesium-137.
Here are some pictures of radioactive lava:

The caption:
By the way, in the first week of April 1986, the concentration of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere was 349.79 ppm. (The week ending April 6, 1986 from the data at the Mauna Loa CO2 observatory.) In the first week of April 2019, the same figure was 413.13 ppm. Unlike the world wide levels of Cs-137 since the much discussed Fukushima event, the levels of the dangerous fossil fuel waste are going up, not down. Since April 6, 1986, using the figures reported at the Mauna Loa observatory this morning, for the week of February 9, 2020, the concentration of this dangerous fossil fuel waste in the planetary atmosphere has risen by 64.61 ppm.
Unless you are Mr. Zirlin or a colleague involved in the same kind of work, the probability that you will die as a result of Chernobyl is roughly comparable to the probability that you will win the Powerball lottery. The probability that you will die from air pollution, or a car accident, or the effects of eating cows - if you do eat them - is five or six orders of magnitude higher.
Maybe you couldn't care less; maybe you think Chernobyl is the worst thing that ever happened. I disagree. There are, in my mind, hundreds of thousands of things that were worse. For me, since I'm in the unusual position of actually giving a shit about future generations, climate change is much, much, much worse. With respect to nuclear reactors, Chernobyl and Fukushima type events are easily engineered away, just as we engineer away aircraft failures, which by the way, have killed way more people than nuclear reactors have in the last 60 years.
I hope you're having a pleasant Sunday morning.
Extraction and Separation of Lanthanides Using Hydrophobic Ionic Liquids.
The paper I'll discuss in this post is this one: Synergistic Enhancement of the Extraction and Separation Efficiencies of Lanthanoid(III) Ions by the Formation of Charged Adducts in an Ionic Liquid (Hiroyuki Okamura et al. Ind. Eng. Chem. Res. 2020, 59, 1, 329-340).
The importance of the lanthanide elements to modern technology cannot be over estimated. Among many other systems, their importance to so called "renewable energy," particularly the wind industry, is dependent on access to these elements since the wind industry depends on the use of permanent magnets, which are in turn, dependent access to the elements neodymium and dysprosium. The majority of these elements are obtained in China, often under appalling environmental and health and safety conditions.
I'll jump here to the text of the paper which has a nice summary of the value of these elements:
Recently, ionic liquids (ILs) have attracted considerable interest in green and sustainable chemistry and engineering(10,11) and in applications such as functional materials,(12,13) catalysts,(14?16) and pharmaceuticals.(17)Typical ILs are composed of an asymmetric and bulky organic cation and a halide-containing inorganic or organic anion. The ILs consist entirely of ions, and their properties, such as extremely low vapor pressure and incombustibility, are thus quite different from those of molecular diluents. The physicochemical properties of ILs uniquely depend on both the cation and anion.(18) Thus, the polarity, hydrophobicity, and miscibility can be tuned by varying the constituent ions. These unique features offer ILs great potential as functional extraction media for solvent extraction.(19,20)...
The authors discuss, with references, to some of the complexing agent classes that have been utilized in the current organic solvent technology that is currently used in this technology, and looks at some different members of this class for use in their ionic liquid.
The structures of these components are shown:

The caption:
The "Htta" reagent, (2-thenoyltrifluoroacetone) is an acidic reagent, and as such, its properties vary with pH. It has been widely utilized in the extraction chemistry of europium, a feature to which I'll allude below. "TOPO" is trioctylphosphine oxide.
The authors discuss their approach using these reagents.
Some experimental details:
Some results are shown graphically:


Since the DU editor no longer allows exponents, and because the captions are quite busy, captions for figures 4 through 7 are posted as graphics objects. A cubic decimeter (dm^(-3) is a fancy word (a bow to SI units) for "liter.”

The caption for the above figure:


The caption for the above figure:


The caption for the above figure:

This next graphic is a 3D representation of the synergistic effects of the two extractant agents TOPO and htta-

The caption for the above figure:

Of course the interesting thing about this system is that not only can it extract the lanthanides, but can do so in an environment which also allows for their separation.
This graphic shows an example:

The caption:
The structure of the complexes was investigated, and showed, for europium, that a

The lower lanthanides, from lanthanum up to and including gadolinium are also components of used nuclear fuels, and the fast separation and recovery of these elements is desirable. The high energy density of nuclear fuels means that the amount of these elements obtainable from nuclear fuels is low compared to natural sources, but they still have economic value, and those which retain significant radioactivity over relatively long periods of time, significantly samarium and europium, may have particularly high value to accomplish certain environmentally important tasks, owing to this activity.
It is interesting to note that samarium and europium, along with cerium are the lanthanides which exhibit multiple oxidation states. Europium has a well known +2 oxidation state, stable in aqueous solution, samarium a +2 oxidation state in the solid phase and non-aqueous solution, and cerium a +4 oxidation state under a wide variety of conditions, making it useful for thermochemical water and carbon dioxide splitting among many other redox situations, including self cleaning ovens. These three elements are also the three lanthanides in nuclear fuel whose radioactivity remains for appreciable periods of times, samarium for a few centuries owing to its 151 isotope (t(1/2) = 88.8 years), europium for about a century owing to its 152 isotope (t(1/2) = 13.5 years) and 154 isotope (t(1/2 = 8.5 years), and cerium for less than a decade (t(1/2) = 284.9 days). The quantities of samarium and europium isotopes are generally low because of the high neutron capture cross sections of the isotopes of these elements. (Pure non-radioactive europium can be obtained by allowing samarium 151 isolated from used nuclear fuel to decay into this stable daughter nuclide.) Although europium is a valuable element, and tends to be somewhat depleted in many lanthanide ores, the small amounts possible to isolate from decayed samarium-151 is small, and not likely to have tremendous economic value.
It is not clear how this system might operate in the separation of used nuclear fuels to recover the valuable constituents. The radiation stability of ionic liquids has been extensively studied, notably by a scientist whose work I follow quite closely, Jim Wishart, at Brookhaven National Lab and also by his frequent co-author, Ilya Shkrob at Argonne National Labs. The imidazolium ions are known to degrade in radiation fields, albeit (as Wishart has noted) not necessarily at a rate that effects its performance as a solvent, apparently because of the ability to solvate electrons. (I became familiar with Dr. Wishart when attending a lecture of his on electron solvation.) In some systems, degradants of the widely used TBP (tributyl phosphate) extractant in existing nuclear fuel reprocessing schemes can give rise Zr and Pu (and other metals) upon degradation. The TOPO reagent is sort of an analogue, although TBP is a phosphate and TOPO is a phospine. Htta has been used as an extractant for other metals as well.
However, the wonderful thing about ionic liquids is that their composition is tunable to fit purposes. There is an entire class of ionic liquids that use phosphinium ions as cations, by the way. Another feature is that, being ionic, they are useful for the performance of electrochemistry. Owing to the aforementioned variable oxidation states of cerium, samarium, and europium, it is possible to imagine very fast facile separations of these elements by exploiting solubility differences between oxidation states, as well as extraction into liquid metal cathodes or deposition on solid electrodes.
This paper is certainly not the last word in these types of separations, but it's a lovely paper along a route to a sustainable world.
I hope you're enjoying your weekend.
Fiber Supported Amino Acidate Functionalized Ionic Liquid Gels for Direct Air CO2 Capture.
The paper I'll discuss in this post is this one: Hollow Fiber-Type Facilitated Transport Membrane Composed of a Polymerized Ionic Liquid-Based Gel Layer with Amino Acidate as the CO2 Carrier (Hideto Matsuyama et al. Ind. Eng. Chem. Res. 2020, 59, 5, 2083-2092)
This paper caught my eye because it has in its introductory text a "by 2100" statement that's quite different than all those I've been hearing my whole adult life about so called "renewable energy." I first started hearing these when I was effectively a child - since I was a gullible sort well into my twenties - about how "by 2000" we'd live in a renewable energy nirvana.
We don't.
The fastest growing source of energy on this planet in this century has been coal, despite all the "coal is dead" rhetoric that flies around among the other distortions one hears in these times of the celebration of the lie, Trumpian and otherwise. So called "renewable energy" remains what it has been since the early 20th century (when its abandonment was nearly complete), a trivial form of energy.
One doesn't see much blunt realism, even in the primary scientific literature, but this paper has it. To wit, from the introductory text:
I have put in bold the realistic statement.
It's realistic because we are no where near close to doing anything effective to address climate change. We'd rather prattle on endlessly about Fukushima - without recognizing that almost all of the people in the area of the failed reactors who died were killed by seawater and not radiation - than we would have a serious discussion of what the destruction of the entire planetary atmosphere might mean.
980 ppm sounds reasonable to me. In my lifetime, I've seen an increase of over 100 ppm, and despite the trillions thrown at so called "renewable energy" the rate of increase (the second derivative) is rising and accelerating (the third derivative).
I have been studying and thinking about direct air capture for sometime to dream that something will be available for future generations to clean up the mess we left for them because, well, we need our cars, and we need our vacations, and we need our suburbs, etc, etc.
Fugettaboutit.
The technical stuff from the paper:
The introduction continues thus:
However, CO2 desorption requires high temperatures, which increases equipment costs and energy consumption. On the other hand, the membrane technology does not require high temperatures for CO2 separation, and energy-efficient processes can be established. In particular, facilitated transport membranes (FTMs) are suitable for capturing CO2 from gases with low CO2 concentration, such as those found in closed spaces (0.5–0.6% of CO2) as well as flue gases (approximately 10–15% of CO2). Therefore, FTMs are suitable for DAC applications.
FTMs are functional membranes that contain a chemical compound called a CO2 carrier.(8?24) The CO2 carrier can selectively and reversibly absorb CO2 by a chemical reaction. Therefore, FTMs have an extremely high CO2 permeability, even at low CO2 partial pressure.(8?12,16?24) Sarma Kovvali et al. reported that FTMs consisting of polyamidoamine in a porous hydrophilized polyvinylidene fluoride flat membrane showed CO2 permeability of 4100 barrer [1 barrer = 1 × 10–10 cm3 (STP) cm/(cm2 s cmHg)] at a CO2 partial pressure of 0.26 cmHg for completely humidified CO2/N2 mixed gas at room temperature.(10) Chen et al. also reported that FTMs containing glycine–Na–glycerol had CO2 permeability of more than 3000 barrer at CO2 partial pressure of 0.5 cmHg under relative humidity exceeding 70% at room temperature (23 ± 2 °C).(8)...
What follows is a number of references to publications in which various scientific groups discussed the utility of amino acids for CO2 capture.
The authors not however, that the thickness of layers and the viscosity of amino acid solutions are a limitation. They here suggest a supported membrane consisting of hollow fibers to support an amino acidate (an ionic amino acid species). (This is, by the way, sort of similar to what goes on in biological systems for transporting CO2. Biological systems are quite good at CO2 capture from the air.)
The authors write:
Ionic liquids are comprised of organic ions that are positively charged and organic ions that are negatively charged. (Sometimes one of the ions will not be organic, but most often they are.) These are not entirely new compounds. Stable organic ions have been known for a very long time. Brains, among other organs, function because of the organic ion choline, which is positively charged, and many choline based ionic liquids are known. These ionic salts are remarkable because, as the name implies, they can be liquid at, below or slightly above room temperature. They are a positively huge area of research.
Some pictures from the text to illuminate the authors approach in which they polymerize a fairly well known class of organic cations, alkyl imidazolium cations, and then do ion exchange with the resulting resin, exchanging a bromine ion for a glycinate anion, derived from the simplest amino acid, glycine:

The caption:

The caption:

The caption:

The caption:
The separation between nitrogen and carbon dioxide - the important point since air is mostly nitrogen - as a function of gel layer thickness is shown:

The caption:
(A GPU is a unit of gas permeance that has a unit of volume of a gas at standard temperature and pressure (STP) per unit of surface area of the permeating surface, per second per unit of pressure). The unit is sometimes denoted the "Barrer." )
Subsequent diagrams will make better sense with this bit of text:

The caption:
The molecular weight distribution was determined by old fashioned GPC (Size exclusion chromatography) and not something like MALS. (Multiangle light scattering) It's good as a first approximation.
Some results of the dialysis:

The caption:

The caption:

The caption:
And now the important stuff, the selectivity:

The caption:
The conclusion:
These are dark times, and in dark times, sometimes it relieves the pain to recognize that for all that is, there are also things - good things - that are possible.
We have left our children nothing but disaster, except, in the cases like the work of scientists like these, perhaps some tools that they might use to dig out of the graves we have dug for them.
Have a nice TGIF day tomorrow.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,542